Eha Lotion: Package Insert / Prescribing Info
Package insert / product label
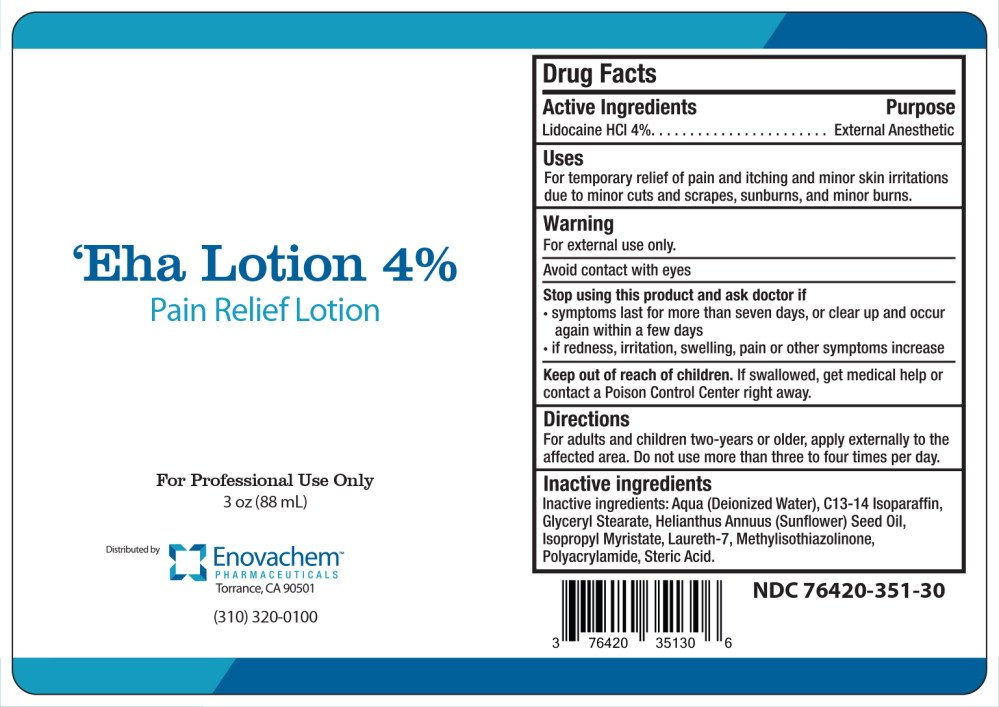
Generic name: lidocaine hydrochloride
Dosage form: lotion
Drug class: Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Jan 20, 2025.
On This Page
Indications and Usage for Eha Lotion
For temporary relief of pain and itching and minor skin irritations due to minor cuts and scrapes, sunburns, and minor burns.
Warnings
For external use only.
Avoid contact with eyes
Related/similar drugs
Eha Lotion Dosage and Administration
For adults and children two-years or older, apply externally to the affected area. Do not use more than three to four times per day.
| EHA
lidocaine hydrochloride lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Asclemed USA, Inc. (059888437) |
More about Eha Lotion (lidocaine topical)
- Check interactions
- Compare alternatives
- Latest FDA alerts (8)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical anesthetics
- Breastfeeding
Professional resources
Other brands
Lidocaine Viscous, Lidocan Patch, ZTlido, Xylocaine Jelly, ... +34 more