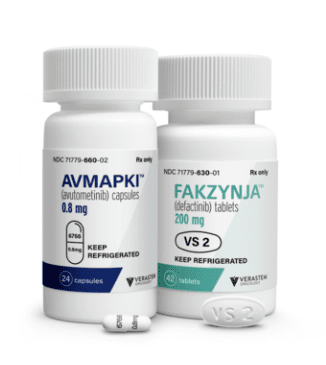
Amapki Fakzynja Co-Pack: Package Insert / Prescribing Info
Package insert / product label
Generic name: avutometinib potassium and defactinib hydrochloride
Dosage form: kit
Medically reviewed by Drugs.com. Last updated on May 26, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
AVMAPKI™ FAKZYNJA™ CO-PACK (avutometinib capsules; defactinib tablets), co-packaged for oral use
Initial U.S. Approval: 2025
Indications and Usage for Amapki Fakzynja Co-Pack
AVMAPKI FAKZYNJA CO-PACK, a combination of avutometinib and defactinib, each kinase inhibitors, is indicated for the treatment of adult patients with KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) who have received prior systemic therapy.
This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. (1,14)
Amapki Fakzynja Co-Pack Dosage and Administration
Dosage Forms and Strengths
Contraindications
None. (4)
Warnings and Precautions
- Ocular Toxicities: Ocular toxicities, including visual impairment and vitreoretinal disorders, occurred. Perform comprehensive ophthalmic evaluation at baseline, prior to cycle 2, every three cycles thereafter, and as clinically indicated. Withhold AVMAPKI FAKZYNJA CO-PACK for ocular toxicities until improvement at the same or reduced dose. Permanently discontinue AVMAPKI FAKZYNJA CO-PACK for any grade 4 toxicity. (2.4, 5.1)
- Serious Skin Toxicities: Skin toxicities, including photosensitivity and severe cutaneous adverse reactions (SCARs), occurred. Adhere to concomitant medications. Monitor for skin toxicities and interrupt, reduce or permanently discontinue AVMAPKI FAKZYNJA CO-PACK based on severity, tolerability and duration. (2.4, 5.2)
- Hepatotoxicity: Monitor liver function tests prior to each cycle, on day 15 of the first 4 cycles, and as clinically indicated. Withhold, reduce or discontinue AVMAPKI FAKZYNJA CO-PACK based on severity and persistence of abnormality. (2.4, 5.3)
- Rhabdomyolysis: Monitor creatine phosphokinase prior to the start of each cycle, on day 15 of the first four cycles, and as clinically indicated. If increased CPK occurs, evaluate patients for rhabdomyolysis or other causes. Withhold, reduce or permanently discontinue AVMAPKI FAKZYNJA CO-PACK based on severity and duration of the adverse reaction. (2.4, 5.4)
- Embryo-Fetal Toxicity: AVMAPKI FAKZYNJA CO-PACK can cause fetal harm. Advise patients of the potential risk to a fetus and to use effective contraception. (5.5, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common (≥ 25%) adverse reactions, including laboratory abnormalities, were increased creatine phosphokinase, nausea, fatigue, increased aspartate aminotransferase, rash, diarrhea, musculoskeletal pain, edema, decreased hemoglobin, increased alanine aminotransferase, vomiting, increased blood bilirubin, increased triglycerides, decreased lymphocyte count, abdominal pain, dyspepsia, dermatitis acneiform, vitreoretinal disorders, increased alkaline phosphatase, stomatitis, pruritus, visual impairment, decreased platelet count, constipation, dry skin, dyspnea, cough, urinary tract infection, and decreased neutrophil count. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Verastem at 1-833-633-8786 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong and moderate CYP3A4 inhibitors: Avoid concomitant use with AVMAPKI FAKZYNJA CO-PACK. (7.1)
- Strong and moderate CYP3A4 inducers: Avoid concomitant use with AVMAPKI FAKZYNJA CO-PACK. (7.1)
- Warfarin: Avoid concomitant use of AVMAPKI FAKZYNJA CO-PACK with warfarin and use an alternative to warfarin. (7.2)
- Gastric acid reducing agents: Avoid concomitant use of AVMAPKI FAKZYNJA CO-PACK with proton pump inhibitors (PPIs) or H2 receptor antagonists. If use of an acid-reducing agent cannot be avoided, administer FAKZYNJA 2 hours before or 2 hours after the administration of a locally acting antacid. (7.1)
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
Infertility: May impair fertility in males and females. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2025
Full Prescribing Information
1. Indications and Usage for Amapki Fakzynja Co-Pack
AVMAPKI FAKZYNJA CO-PACK is indicated for the treatment of adult patients with KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) who have received prior systemic therapy.
This indication is approved under accelerated approval based on tumor response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
2. Amapki Fakzynja Co-Pack Dosage and Administration
2.1 Patient Selection
Select patients for the treatment of recurrent LGSOC with AVMAPKI FAKZYNJA CO-PACK based on the presence of a KRAS mutation in tumor specimens [see Clinical Studies (14)].
An FDA-approved test for the detection of a KRAS mutation in LGSOC for selecting patients for treatment with AVMAPKI FAKZYNJA CO-PACK is not available.
2.2 Eye Exams and Prophylactic Skin Medications
Ophthalmic Exams
Conduct a comprehensive ophthalmic exam at baseline, prior to cycle 2, and every three cycles thereafter regardless of baseline exam findings, and as clinically indicated [see Warnings and Precautions (5.1)].
Prophylactic Medications for Skin Reactions
With initiation of and during at least the first 2 cycles of AVMAPKI FAKZYNJA CO-PACK administer [see Warnings and Precautions (5.2)]:
- Topical corticosteroid (applied to the face, scalp, neck, upper chest and upper back)
- Systemic oral antibiotics
2.3 Recommended Dosage and Administration
AVMAPKI Capsules
The recommended dosage of AVMAPKI capsules is 3.2 mg (four 0.8 mg capsules) taken orally twice weekly (Day 1 and Day 4) for the first 3 weeks of each 4-week cycle until disease progression or unacceptable toxicity.
Take AVMAPKI at the same time with each dose. AVMAPKI should be taken with food [see Clinical Pharmacology (12.3)]. Swallow capsules whole. Do not chew, break, or open the capsules.
If a dose of AVMAPKI is missed by more than 24 hours, skip the missed dose and take the next scheduled dose as prescribed. Do not take two doses at the same time to make up for a missed dose. If vomiting occurs after taking AVMAPKI, do not take an additional dose. Take the next scheduled dose as prescribed.
FAKZYNJA Tablets
The recommended dosage of FAKZYNJA tablets is 200 mg (one tablet) taken orally twice daily for the first 3 weeks of each 4-week cycle until disease progression or unacceptable toxicity.
Take each dose of FAKZYNJA with food [see Clinical Pharmacology (12.3)]. Swallow tablets whole. Do not chew, break or crush the tablets.
If a dose of FAKZYNJA is missed by more than 6 hours, skip the missed dose and take the next scheduled dose as prescribed. Do not take two tablets at the same time to make up for a missed dose. If vomiting occurs after taking FAKZYNJA, do not take an additional dose. Take the next scheduled dose as prescribed.
2.4 Dosage Modifications for Adverse Reactions
Dose reductions due to adverse reactions due to AVMAPKI FAKZYNJA CO-PACK are summarized in Table 1.
| Dose Level | AVMAPKI Capsule | FAKZYNJA Tablet |
|---|---|---|
| Starting dose | 3.2 mg twice weekly for first 3 weeks of each 4-week cycle | 200 mg twice daily for first 3 weeks of each 4-week cycle |
| Dose reduction | 2.4 mg twice weekly for first 3 weeks of each 4-week cycle | 200 mg once daily for first 3 weeks of each 4-week cycle |
| Permanently discontinue both AVMAPKI and FAKZYNJA in patients unable to tolerate after one dose reduction of both products. | ||
Dosage modifications for adverse reactions to AVMAPKI FAKZYNJA CO-PACK are summarized in Table 2.
| Adverse Reaction | Severity* | Dose Modification |
|---|---|---|
| N/A: Not applicable | ||
|
||
| Keratitis
[see Warnings and Precautions (5.1)] | Confluent superficial keratitis, a cornea epithelial defect, or 3-line or more loss in best corrected distance visual acuity | Withhold AVMAPKI FAKZYNJA CO-PACK until resolved to nonconfluent superficial keratitis, then resume at same dose. |
| Corneal ulcer or stromal opacity or best corrected distance visual acuity 20/200 or worse Corneal perforation | Withhold AVMAPKI FAKZYNJA CO-PACK until resolved to nonconfluence superficial keratitis, then resume at reduced dose. Permanently discontinue AVMAPKI FAKZYNJA CO-PACK. |
|
| Blurred vision
[see Warnings and Precautions (5.1)] | BCVA worse than baseline but no worse than 20/200 | Withhold AVMAPKI FAKZYNJA CO-PACK until resolution to baseline or 20/40, whichever is worse, then resume treatment at same dose. |
| BCVA 20/200 or worse | Withhold AVMAPKI FAKZYNJA CO-PACK until resolution to baseline or 20/40, whichever is worse, then resume at reduced dose. | |
| Conjunctivitis
[see Warnings and Precautions (5.1)] | Confluent superficial punctate staining, moderate to severe vasodilation | Withhold AVMAPKI FAKZYNJA CO-PACK until resolution to nonconfluent superficial keratitis, then resume at same dose. |
| Conjunctival ulcer or neovascularization | Withhold AVMAPKI FAKZYNJA CO-PACK until resolution to nonconfluent superficial keratitis, then resume at reduced dose. | |
| Retinal Pigment Epithelial (RPE) Detachment
[see Warnings and Precautions (5.1)] | N/A | First occurrence
|
| Rash
[see Warnings and Precautions (5.2)] | Grade ≤ 2 | Consider withholding AVMAPKI FAKZYNJA CO-PACK if rash does not respond to supportive care or recurs after resolution to Grade ≤1. Dose reduce AVMAPKI FAKZYNJA CO-PACK for intolerable Grade 2. |
| Grade 3 | Withhold AVMAPKI FAKZYNJA CO-PACK until resolved to Grade 2 then resume at reduced dose. Resume at same dose if resolved to Grade ≤1. Permanently discontinue AVMAPKI FAKZYNJA CO-PACK for recurrent Grade 3 despite dose reduction. |
|
| Grade 4 | Permanently discontinue AVMAPKI FAKZYNJA CO-PACK. | |
| Hepatotoxicity
[see Warnings and Precautions (5.3)] | Grade 2 | Withhold AVMAPKI FAKZYNJA CO-PACK for
|
| Grade 3 | Withhold AVMAPKI FAKZYNJA CO-PACK
|
|
| Grade 4 | Withhold AVMAPKI FAKZYNJA CO-PACK for
|
|
| Increased Blood Creatine Phosphokinase (CPK)
[see Warnings and Precautions (5.4)] | Grade 3 | Withhold AVMAPKI FAKZYNJA CO-PACK, if improves to Grade ≤1 within three weeks resume at same dose. Permanently discontinue AVMAPKI FAKZYNJA CO-PACK for CPK elevation longer than three weeks. |
| Grade 4 | Withhold AVMAPKI FAKZYNJA CO-PACK, if improves to Grade ≤1 within three weeks resume at reduced dose. Permanently discontinue AVMAPKI FAKZYNJA CO-PACK for CPK elevation longer than three weeks. |
|
| Any grade CPK elevation with rhabdomyolysis or other event related to CPK elevation | Permanently discontinue AVMAPKI FAKZYNJA CO-PACK | |
| Other Adverse Reactions | Grade 2 | Consider withholding AVMAPKI FAKZYNJA CO-PACK if adverse reaction does not respond to supportive care or recurs after resolution to Grade ≤1. |
| Grade 3 | First occurrence:
Withhold AVMAPKI FAKZYNJA CO-PACK until resolved to baseline or Grade ≤1 then resume at same dose. Second occurrence: Withhold AVMAPKI FAKZYNJA CO-PACK until resolved to baseline or Grade ≤1 then resume at reduced dose. Permanently discontinue AVMAPKI FAKZYNJA CO-PACK for recurrent Grade 3 despite dose reduction. |
|
| Grade 4 | Permanently discontinue AVMAPKI FAKZYNJA CO-PACK. | |
3. Dosage Forms and Strengths
AVMAPKI FAKZYNJA CO-PACK is AVMAPKI (avutometinib) capsules co-packaged with FAKZYNJA (defactinib) tablets.
- AVMAPKI capsules contain 0.8 mg avutometinib and are white capsules with "6766" printed on the cap and the strength "0.8 mg" printed on the body in black ink.
- FAKZYNJA tablets contain 200 mg defactinib and are white to off-white tablets, oval and debossed with "VS2" on one side.
5. Warnings and Precautions
5.1 Ocular Toxicities
AVMAPKI FAKZYNJA CO-PACK can cause ocular adverse reactions, including visual impairment and vitreoretinal disorders.
Ocular adverse reactions occurred in 68% of patients with recurrent LGSOC treated with AVMAPKI FAKZYNJA CO-PACK. Common ocular adverse reactions (≥ 5%) were visual impairment (38%), dry eye (13%), orbital/periorbital edema (8%), and vitreous floaters (5%). Thirty-five patients (26%) experienced vitreoretinal disorders, including retinal detachment (9%), and retinal vein occlusion (0.7%). Eighteen patients (13%) experienced an ocular adverse reaction that resulted in dose interruption of AVMAPKI FAKZYNJA CO-PACK and one patient experienced an ocular adverse reaction that resulted in dose reduction.
The median time to onset of symptomatic ocular adverse reactions was 5 days (range 1 to 943 days) and to onset of asymptomatic ocular adverse reactions was 112 days (range 23 to 943 days). The median time to onset of retinal detachment was 27 days (range 2 to 535 days). Of the patients who experienced ocular adverse reactions, 29% had ongoing ocular events at last follow-up.
Refer patients to a qualified eye care professional for a comprehensive ophthalmic exam at baseline, prior to cycle 2, every three cycles thereafter, and as clinically indicated. Promptly refer patients to an eye care professional for any new or worsening ocular signs or symptoms.
Monitor for ocular adverse reactions and withhold, reduce, or permanently discontinue AVMAPKI FAKZYNJA CO-PACK based on severity and persistence of ocular adverse reactions [see Dosage and Administration (2.4)].
5.2 Serious Skin Toxicities
AVMAPKI FAKZYNJA CO-PACK can cause serious skin toxicities, including Severe Cutaneous Adverse Reactions (SCARs). Cases of acute generalized exanthematous pustulosis, erythema multiforme and drug reaction with eosinophilia and systemic symptoms have been reported in clinical trials of avutometinib (a drug in AVMAPKI FAKZYNJA CO-PACK).
Skin toxicities occurred in 94% of patients with recurrent LGSOC treated with AVMAPKI FAKZYNJA CO-PACK. The most common skin toxicities (≥ 10%) were rash (67%), dermatitis acneiform (43%), dry skin (43%), pruritus (32%), and photosensitivity (13%). Grade 3 skin reactions occurred in 12% of patients including dermatitis acneiform (7%), rash (7%), and pruritus (1.5%). Thirteen patients (10%) developed bacterial skin infections. Skin toxicity led to dose interruption of AVMAPKI FAKZYNJA CO-PACK in 10%, to dose reduction in 7%, and to permanent discontinuation in 0.7% of patients. The median time to onset of the first skin toxicity was 14 days (range 1 to 500 days). At last follow-up, 66% of patients had ongoing skin toxicity.
Patients in RAMP-201 used topical corticosteroids (applied to the face, scalp, neck, upper chest and upper back) and systemic oral antibiotics for prophylaxis of skin adverse reactions. These medications were initiated at the start of AVMAPKI FAKZYNJA CO-PACK and administered during at least the first two cycles of treatment.
Limit unnecessary exposure to sunlight and apply daily sunscreen (sun protection factor [SPF] ≥ 30). Monitor for skin toxicity and withhold, reduce dose, or permanently discontinue AVMAPKI FAKZYNJA CO-PACK based on severity and persistence [see Dosage and Administration (2.4)].
5.3 Hepatotoxicity
AVMAPKI FAKZYNJA CO-PACK can cause hepatotoxicity.
In patients with recurrent LGSOC who received AVMAPKI FAKZYNJA CO-PACK, increased AST (73%), bilirubin (51%), ALT (49%), and alkaline phosphatase (46%) occurred. Grade 3-4 elevations in ALT was 3%, AST was 3%, bilirubin was 2.3% and alkaline phosphatase was 0.8%. Elevations in one or more liver related laboratory values led to dose interruption for 20%, dose reduction for 2.2%, and permanent discontinuation for 0.7% of patients.
Increased blood bilirubin may be attributed to defactinib (a component of AVMAPKI FAKZYNJA CO-PACK) due to the inhibition of enzymes responsible for metabolizing (uridine diphosphate-glucuronosyltransferase (UGT)1A1) and transporting (Organic Anion Transporting Polypeptides (OATP)1B1/1B3) bilirubin [see Clinical Pharmacology (12.3)].
Monitor liver related laboratory values prior to the start of each cycle, on day 15 of the first four cycles, and as clinically indicated. Withhold, reduce dose, or permanently discontinue AVMAPKI FAKZYNJA CO-PACK based on severity and duration of these adverse reactions [see Dosage and Administration (2.4)].
5.4 Rhabdomyolysis
AVMAPKI FAKZYNJA CO-PACK can cause increased creatine phosphokinase (CPK). Increased CPK occurred in 75% of patients with recurrent LGSOC treated with AVMAPKI FAKZYNJA CO-PACK, including Grade 3-4 elevations in 18% of patients. Among the patients who experienced an elevation in CPK, concurrent increase in creatinine occurred in 19% (n=19/102) and myalgia occurred in 10% (n=10/102). Elevation of CPK >10 times the baseline value with a concurrent increase in serum creatinine of ≥1.5 times the baseline value occurred in 0.7% of patients. Increased CPK resulted in dose interruption for 22%, in dose reduction for 7%, and in discontinuation for 2.9% of patients. Rhabdomyolysis has occurred in a patient with LGSOC treated with AVMAPKI FAKZYNJA CO-PACK at the recommended dosage in a clinical trial.
Monitor CPK prior to the start of each cycle, on day 15 of the first four cycles, and as clinically indicated. If increased CPK occurs, evaluate patients for rhabdomyolysis or other causes. Withhold, reduce or permanently discontinue AVMAPKI FAKZYNJA CO-PACK based on severity and duration of the adverse reactions [see Dose Modifications (2.4)].
5.5 Embryo-Fetal Toxicity
Based on the mechanisms of action, AVMAPKI FAKZYNJA CO-PACK can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. Inhibition of either molecular pathway has been associated with embryo-fetal anomalies and lethality in animals.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with AVMAPKI FAKZYNJA CO-PACK and for 1 month after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with AVMAPKI FAKZYNJA CO-PACK and for 4 months after the last dose [see Use in Specific Populations (8.3)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Ocular Toxicities [see Warnings and Precautions (5.1)]
- Serious Skin Toxicities [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.3)]
- Rhabdomyolysis [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in Warnings and Precautions reflect exposure to the AVMAPKI FAKZYNJA CO-PACK (combination of AVMAPKI 3.2 mg twice weekly and FAKZYNJA 200 mg twice daily) for the first 3 weeks in a 4-week cycle until disease progression or unacceptable toxicity in 136 adult patients with recurrent LGSOC treated on RAMP-201 and FRAME (NCT03875820). The median duration of treatment was 10 months (range 0 to 51 months).
RAMP-201
The safety of AVMAPKI FAKZYNJA CO-PACK was evaluated in RAMP-201, a single-arm multicenter trial in 57 patients with KRAS-mutated recurrent LGSOC [see Clinical Studies (14)]. Patients received AVMAPKI FAKZYNJA CO-PACK (AVMAPKI 3.2 mg twice weekly and FAKZYNJA 200 mg twice daily) for the first 3 weeks in a 4-week cycle until disease progression or unacceptable toxicity. The median duration of treatment was 12 months (range 0.03-40).
Serious adverse reactions occurred in 32% of patients who received AVMAPKI FAKZYNJA CO-PACK. The most common (≥2%) serious adverse reactions were sepsis (9%), intestinal obstruction (3.6%), pyelonephritis (3.6%), and hydronephrosis (3.6%). Fatal adverse reactions occurred in 3.6% of patients who received AVMAPKI FAKZYNJA CO-PACK, including intestinal obstruction (1.8%) and perforation (1.8%).
Permanent discontinuation of AVMAPKI FAKZYNJA CO-PACK due to an adverse reaction occurred in 14% of patients. The adverse reactions leading to permanent discontinuation included elevations in creatine phosphokinase, dyspnea, malaise, decreased glomerular filtration rate, hyperbilirubinemia, increased alanine aminotransferase, and abdominal pain (1.8% each).
Dosage interruptions of AVMAPKI FAKZYNJA CO-PACK due to an adverse reaction occurred in 84% of patients. Adverse reactions which required dosage interruptions in ≥ 5% of patients included elevations in creatine phosphokinase (25%), hyperbilirubinemia (25%), diarrhea (12%), edema (11%), fatigue (9%), vision blurred (9%), vitreoretinal disorders (7%), transaminitis (7%), paronychia (5%), nausea (5%), abdominal pain (5%), vomiting (5%), dyspnea (5%), sepsis (5%), and rash (5%).
Dose reductions of AVMAPKI FAKZYNJA CO-PACK due to an adverse reaction occurred in 44% of patients. Adverse reactions which required dose reductions in ≥ 5% of patients were elevations in creatine phosphokinase (9%), fatigue (5%), hyperbilirubinemia (5%), and dermatitis acneiform (5%).
The most common (≥25%) adverse reactions, including laboratory abnormalities, were increased creatine phosphokinase, nausea, fatigue, increased aspartate aminotransferase, rash, diarrhea, musculoskeletal pain, edema, decreased hemoglobin, increased alanine aminotransferase, vomiting, increased blood bilirubin, increased triglycerides, decreased lymphocyte count, abdominal pain, dyspepsia, dermatitis acneiform, vitreoretinal disorders, increased alkaline phosphatase, stomatitis, pruritus, visual impairment, decreased platelet count, constipation, dry skin, dyspnea, cough, urinary tract infection, and decreased neutrophil count.
Table 3 summarizes the adverse reactions in RAMP-201.
| Adverse Reaction | AVMAPKI FAKZYNJA CO-PACK N = 57 |
|
|---|---|---|
| All Grades % | Grade 3 or 4†
% |
|
|
||
| Gastrointestinal disorders | ||
| Nausea | 74 | 1.8 |
| Diarrhea | 68 | 7 |
| Vomiting | 49 | 3.5 |
| Abdominal pain‡ | 39 | 1.8 |
| Dyspepsia‡ | 37 | 0 |
| Stomatitis‡ | 35 | 3.5 |
| Constipation | 30 | 0 |
| Dry mouth | 18 | 0 |
| Decreased Weight | 11 | 0 |
| General disorders and administration site condition | ||
| Fatigue‡ | 72 | 3.5 |
| Edema‡ | 67 | 1.8 |
| Skin and subcutaneous tissue disorders | ||
| Rash§ | 72 | 3.5 |
| Dermatitis acneiform¶ | 37 | 5.3 |
| Pruritus‡ | 35 | 1.8 |
| Dry skin‡ | 30 | 0 |
| Alopecia‡ | 23 | 0 |
| Photosensitivity‡ | 16 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain‡ | 68 | 1.8 |
| Joint swelling | 11 | 0 |
| Eye disorders | ||
| Vitreoretinal disorders# | 37 | 3.5 |
| Visual impairmentÞ | 35 | 0 |
| Dry eye | 12 | 0 |
| Respiratory disorders | ||
| Dyspnea‡ | 26 | 5.3 |
| Cough | 25 | 0 |
| Nervous system disorders | ||
| Dizziness | 23 | 1.8 |
| Headache | 16 | 0 |
| Neuropathy peripheral‡ | 14 | 0 |
| Dysgeusia | 11 | 0 |
| Vascular disorders | ||
| Hemorrhage‡ | 23 | 0 |
| Hypertension | 16 | 5.3 |
| Venous thromboembolism‡ | 14 | 5.3 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 18 | 1.8 |
| Infections and infestations | ||
| Urinary tract infection | 25 | 3.5 |
| Paronychia | 14 | 1.8 |
| Upper respiratory tract infection | 11 | 0 |
Clinically relevant adverse reactions in < 10% of patients who received AVMAPKI FAKZYNJA CO-PACK included urticaria and decreased ejection fraction.
Table 4 summarizes the laboratory abnormalities in RAMP-201.
| Laboratory Abnormality | AVMAPKI FAKZYNJA CO-PACK | |
|---|---|---|
| All Grades (%)* | Grade 3 or 4 (%)* |
|
|
||
| Chemistry | ||
| Increased creatine phosphokinase | 82 | 19 |
| Increased aspartate aminotransferase | 70 | 3.5 |
| Decreased albumin | 70 | 0 |
| Increased alanine aminotransferase | 58 | 3.5 |
| Increased blood bilirubin | 48 | 3.5 |
| Increased triglycerides | 46 | 3.5 |
| Increased alkaline phosphatase | 37 | 1.8 |
| Decreased potassium | 23 | 9 |
| Hematology | ||
| Decreased hemoglobin | 65 | 5 |
| Decreased lymphocyte count | 40 | 1.8 |
| Decreased platelet count | 35 | 0 |
| Decreased neutrophil count | 25 | 1.8 |
| Urine | ||
| Proteinuria | 22 | 4.4 |
Clinically relevant laboratory abnormalities in < 10% of patients who received AVMAPKI FAKZYNJA CO-PACK included increased INR and prolonged activated partial thromboplastin time.
Related/similar drugs
7. Drug Interactions
7.1 Effect of Other Drugs on AVMAPKI FAKZYNJA CO-PACK
Strong and Moderate CYP3A4 Inhibitors
Avoid concomitant use of AVMAPKI FAKZYNJA CO-PACK with strong or moderate CYP3A4 inhibitors.
Defactinib is a CYP3A4 substrate. Concomitant use of defactinib with a strong CYP3A4 inhibitor increases defactinib exposure [see Clinical Pharmacology (12.3)], which may increase the risk of AVMAPKI FAKZYNJA CO-PACK adverse reactions.
Strong and Moderate CYP3A4 Inducers
Avoid concomitant use of AVMAPKI FAKZYNJA CO-PACK with strong or moderate CYP3A4 inducers.
Defactinib is a CYP3A4 substrate. Concomitant use of defactinib with a strong CYP3A4 inducer decreases defactinib exposure, which may reduce the effectiveness of FAKZYNJA [see Clinical Pharmacology (12.3)].
Gastric Acid Reducing Agents
Avoid concomitant use of AVMAPKI FAKZYNJA CO-PACK with proton pump inhibitors (PPIs) or H2 receptor antagonists. If concomitant use of an acid-reducing agent cannot be avoided, administer FAKZYNJA 2 hours before or 2 hours after the administration of a locally acting antacid.
Concomitant use of FAKZYNJA with gastric acid reducing agents decreases defactinib exposure, which may reduce the effectiveness of AVMAPKI FAKZYNJA CO-PACK [see Clinical Pharmacology (12.3)].
7.2 Effect of AVMAPKI FAKZYNJA CO-PACK on Other Drugs
Warfarin
Avoid concomitant use of AVMAPKI FAKZYNJA CO-PACK with warfarin. For patients requiring anticoagulation, an alternative to warfarin is recommended. If concomitant use is unavoidable, monitor INR frequently during treatment with AVMAPKI FAKZYNJA CO-PACK.
Cases of bleeding and increased INR occurred in patients taking FAKZYNJA concomitantly with warfarin in clinical trials.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on the mechanisms of action, AVMAPKI FAKZYNJA CO-PACK can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of AVMAPKI FAKZYNJA CO-PACK in pregnant women to inform a drug-associated risk. Animal reproductive and developmental toxicity studies have not been conducted with avutometinib or defactinib; however, inhibition of either molecular pathway has been associated with embryo-fetal anomalies and lethality in animals. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of avutometinib, defactinib, or their metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise lactating women not to breastfeed during treatment with AVMAPKI FAKZYNJA CO-PACK and for 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
AVMAPKI FAKZYNJA CO-PACK can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating treatment with AVMAPKI FAKZYNJA CO-PACK.
Contraception
Infertility
Based on animal studies, AVMAPKI FAKZYNJA CO-PACK may impair fertility in females and males of reproductive potential. The effects on fertility were not reversible in animals [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of AVMAPKI FAKZYNJA CO-PACK in pediatric patients has not been established.
8.5 Geriatric Use
Of the 136 patients in clinical studies of AVMAPKI FAKZYNJA CO-PACK, 29% were age 65 years or older. No overall differences in safety were observed between patients age 65 years or older and younger patients. Clinical studies of AVMAPKI FAKZYNJA CO-PACK did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
11. Amapki Fakzynja Co-Pack Description
AVMAPKI FAKZYNJA CO-PACK contains AVMAPKI (avutometinib) capsules co-packaged with FAKZYNJA (defactinib) tablets.
AVMAPKI capsules contain avutometinib, a kinase inhibitor. The chemical name of avutometinib is N-(3-Fluoro-4-{[4-methyl-7-(2-pyrimidinyloxy)-2H-chromen-2-on-3-yl]methyl}-2-pyridyl)-N'-methylsulfamide potassium salt. The molecular formula for avutometinib is C21H17FN5O5S (as potassium salt) and its molecular weight is 509.55. The chemical structure of avutometinib is shown below:
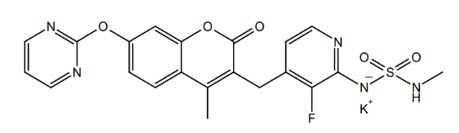
Avutometinib potassium is a white to pale yellow powder. It is practically insoluble in the pH range of 1 to 7 in aqueous media. The pKa is 7.02.
AVMAPKI capsules for oral administration contain 0.8 mg of avutometinib (equivalent to 0.864 mg as the avutometinib potassium salt) with the following inactive ingredients: magnesium stearate and mannitol in a hypromellose capsule shell (carrageenan, hypromellose, potassium chloride, purified water, and titanium oxide) printed with black ink (black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac, and strong ammonia solution).
FAKZYNJA tablets contain defactinib, a kinase inhibitor. The chemical name of defactinib is N-methyl-4-({4-[({3-methyl(methylsulfonyl)aminopyrazin-2-yl}methyl)amino]-5-(trifluoromethyl)pyrimidin-2-yl}amino)benzamide hydrochloride. The molecular formula for defactinib is C20ClH22F3N8O3S (as hydrochloride [HCl] salt) and its molecular weight is 546.96. The chemical structure of defactinib is shown below:
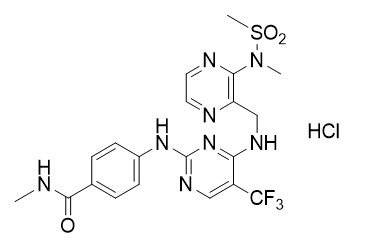
Defactinib hydrochloride is a white to pale yellow powder. It is very slightly soluble at pH 1 and practically insoluble in the pH range of 4.5 to 6.8 in aqueous media. The pKa is 3.81.
FAKZYNJA tablets are available for oral administration. Each FAKZYNJA tablet contains 200 mg defactinib (equivalent to 214.36 mg as the defactinib hydrochloride salt) and the following inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
12. Amapki Fakzynja Co-Pack - Clinical Pharmacology
12.1 Mechanism of Action
Avutometinib
Avutometinib is a MEK1 inhibitor. Avutometinib induces the formation of inactive RAF/MEK complexes and prevents phosphorylation of MEK1/2 by RAF. RAF and MEK proteins are regulators of the RAS/RAF/MEK/ERK (MAPK) pathway. Avutometinib inhibited MEK1/2 and ERK1/2 phosphorylation and proliferation of tumor cell lines harboring KRAS mutations. Treatment of cancer cells with avutometinib increased the level of phosphorylated focal adhesion kinase (FAK).
Defactinib
Defactinib is an inhibitor of FAK and proline-rich tyrosine kinase-2 (Pyk2), the two members of the FAK family of nonreceptor tyrosine kinases. Defactinib inhibited FAK autophosphorylation in cancer cells in vitro and in mouse xenograft models.
Avutometinib in combination with defactinib enhanced inhibition of cell proliferation in vitro and anti-tumor activity in mouse tumor models including LGSOC.
12.2 Pharmacodynamics
Avutometinib exposure-response relationships and the time course of pharmacodynamic response have not been fully characterized.
Defactinib exposure-response relationships and the time course of pharmacodynamic response have not been fully characterized.
12.3 Pharmacokinetics
The pharmacokinetics of avutometinib and defactinib were studied in healthy subjects and in patients with advanced solid tumors and are presented as mean (%CV) unless otherwise specified.
Avutometinib
Avutometinib exhibits dose proportional increases peak plasma concentrations (Cmax) and area under the concentration time curve (AUC) with a single dose ranging from 0.1 mg to 5 mg (0.03 to 1.6 times the approved recommended dose). No significant accumulation of avutometinib was observed at the recommended dosage.
Defactinib
Defactinib exhibits dose proportional increases in Cmax and AUC with twice daily dosing ranging from 12.5 mg to 450 mg (0.06 to 2.25 times the approved recommended dosage). Defactinib steady-state plasma concentrations are reached in approximately 15 days. Defactinib accumulation is approximately 1.5-fold at the approved recommended dosage.
Absorption
The median time to avutometinib peak plasma concentration (Tmax) under fasted conditions is approximately 2 hours.
The median time to defactinib Tmax under fed conditions is approximately 4 hours.
Effect of Food
No clinically significant differences in avutometinib AUC were observed following administration with a high-fat meal. Avutometinib Cmax was decreased by 29% following administration with a high-fat meal (approximately 900 to 1000 calories, 50% fat).
Defactinib AUC increased by 2.7-fold and Cmax increased by 1.9-fold following administration with a high-fat meal (approximately 900 to 1000 calories, 50% fat).
Distribution
Avutometinib steady state apparent volume of distribution (Vd) is 25 L (19%). Avutometinib human plasma protein binding is 99% in vitro.
Defactinib steady state apparent Vd is 1,560 L (59%). Defactinib human plasma protein binding is 90% in vitro.
Elimination
Avutometinib estimated elimination half-life is 51 hours (28%) and the apparent oral clearance (CL/F) is 0.3 L/h (30%).
Defactinib estimated elimination half-life is 9 hours (171%) and the CL/F is 69 L/h (173%).
Metabolism
Avutometinib is primarily metabolized by CYP3A4 and nonenzymatic degradation.
Defactinib is metabolized primarily by CYP3A4 and CYP2C9. Two major metabolites, N-desmethyl sulfonamide (M2) and N-desmethyl amide (M4), were identified in plasma. M2 and M4 AUCs represent 92% and 28% of defactinib exposure, respectively. M2 is inactive and M4 is equipotent when compared to defactinib.
Excretion
After a single dose of radiolabeled avutometinib 2.4 mg (0.8 times the approved recommended dose), 39% (9.5% unchanged) of the dose was recovered in feces and 52% (3.2% unchanged) in urine.
After a single dose of radiolabeled defactinib 400 mg (2 times the approved recommended dose), 87% (52% unchanged) of the dose was recovered in feces and 7.6% (0.8% unchanged) in urine.
Specific Populations
No clinically significant differences in the pharmacokinetics of avutometinib were observed based on age (21 to 87 years), sex, race (84% White, 3% Black and 2% Asian), body weight (40 to 169 kg), mild and moderate renal impairment (CLcr 30 to 89 mL/min, estimated by Cockcroft-Gault), or mild hepatic impairment (AST > ULN or total bilirubin >1 × ULN to 1.5 × ULN). The effect of severe renal impairment (CLcr < 30 mL/min) or moderate to severe hepatic impairment (AST or ALT ≥ 2.5 × ULN or total bilirubin ≥ 1.5 × ULN) on avutometinib pharmacokinetics is unknown.
No clinically meaningful differences in the pharmacokinetics of defactinib were observed based on age (21 to 87 years), sex, race (82% White, 3% Black and 2% Asian), body weight (40 to 169 kg), mild and moderate renal impairment (CLcr 30 to 89 mL/min), or mild hepatic impairment (AST > ULN or total bilirubin >1 × ULN to 1.5 × ULN). The effect of severe renal impairment (CLcr < 30 mL/min) or moderate to severe hepatic impairment (AST or ALT ≥ 2.5 × ULN or total bilirubin ≥ 1.5 × ULN) on defactinib pharmacokinetics is unknown.
Drug Interaction Studies
Clinical Studies
Strong CYP3A4 Inhibitors:
No clinically significant differences in avutometinib pharmacokinetics were observed when used concomitantly with itraconazole (strong CYP3A4 inhibitor).
Defactinib Cmax increased by 2.2-fold and AUC by 3.9-fold following concomitant use with itraconazole (strong CYP3A4 inhibitor) 200 mg daily for 10 days. M4 AUC increased by 2.2-fold and Cmax decreased by 6.8%.
Strong CYP3A4 Inducers:
Avutometinib AUC decreased by 34% with no clinically significant change in Cmax following coadministration with phenytoin (strong CYP3A4 inducer) three times daily for 23 days and a single dose of avutometinib 2.4 mg (0.8 times the approved recommended dose) on Day 17.
Defactinib Cmax decreased by 83% and AUC by 87% following coadministration with phenytoin (strong CYP3A4 inducer) three times daily for 23 days and a single dose of defactinib 200 mg (1.0 times the approved recommended dose) on Day 14. M4 AUC decreased by 79% and Cmax decreased by 70%.
Proton Pump Inhibitors (PPIs):
Defactinib displayed pH-dependent aqueous solubility [see Description (11)]. Defactinib AUC decreased by 79% and Cmax decreased by 85% following concomitant use of multiple doses of omeprazole (PPI) 40 mg daily. M4 AUC decreased by 83% and Cmax decreased by 88%.
In Vitro Studies
CYP450 Enzymes:
Avutometinib is a CYP3A4 substrate, but not a substrate of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6. Avutometinib is not an inhibitor of CYP3A4, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6. Avutometinib is not an inducer of CYP3A4, CYP2B6, and CYP1A2.
Defactinib is a CYP3A4 and CYP2C9 substrate, but not a substrate of CYP1A2, CYP2B6, CYP2C8, CYP2C19, and CYP2D6. Defactinib is a reversible inhibitor of CYP3A4 and CYP2C9, but not CYP1A2, CYP2B6, CYP2C8, CYP2C19, and CYP2D6. Defactinib is a time-dependent inhibitor of CYP3A4. Defactinib is an inducer of CYP2B6, and CYP1A2, but not CYP3A4.
Transporter Systems:
Avutometinib is a substrate of P-gp and BCRP, but not a substrate of MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1 and OCT2. Avutometinib is not an inhibitor of P-gp, BCRP, MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1 and OCT2.
Defactinib is a BCRP and P-gp substrate, but not a substrate of MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1, and OCT2. Defactinib is an inhibitor of P-gp, BCRP, OATP1B1, OATP1B3, and MATE2-K, but not an inhibitor of MATE1, OAT1, OAT3, OCT1, and OCT2.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Avutometinib
Mutagenesis
Avutometinib was not mutagenic in the bacterial reverse mutagenesis (Ames) assay and was not clastogenic in an in vitro Chinese hamster lung chromosome aberration assay or an in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
Dedicated fertility studies have not been conducted with avutometinib. In repeat dose toxicity studies, avutometinib was administered orally once daily for up to 13-weeks duration in rats and monkeys. In female rats, atrophy of the ovary was observed at doses ≥ 0.04 mg/kg/day (approximately ≥ 0.25 times the human exposure at the recommended dose based on AUC). Atrophy of the uterus and thinning of the mucosa and increased single cell necrosis of the mucosal epithelium in the vagina were observed at 0.16 mg/kg/day (0.5 times the human exposure at the recommended dose based on AUC). In female monkeys, thinning of the vaginal mucosa was observed at doses ≥ 0.02 mg/kg/day (≥ 0.7 times the human exposure at the recommended dose based on AUC). All findings were reversible.
Defactinib
Mutagenesis
Defactinib was not mutagenic in the bacterial reverse mutagenesis (Ames) assay and was not clastogenic in an in vitro Chinese hamster lung cell chromosome aberration assay. Defactinib was positive in the in vitro micronucleus assay through an aneugenic mechanism and in the in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
Dedicated fertility studies have not been conducted with defactinib. In male rats administered defactinib for up to 13 weeks, small prostate, seminal vesicle, and testes, decreased prostate weight, diffuse tubule degeneration/atrophy of the testes and sperm granuloma of the epididymis were observed at doses ≥ 125 mg/kg/day (below the human exposure at the recommended dose based on AUC). At the end of the recovery period, findings in the testes were observed.
In male dogs administered defactinib for up to 13 weeks, increased prostate and testes weights and testicular degeneration and cellular debris in the epididymis were observed at doses ≥ 1 mg/kg/day (below the human exposure at the recommended dose based on AUC). At the end of the recovery period, findings in the testes and epididymis that included cellular debris, duct dilation, and hypospermia were observed.
13.2 Animal Toxicology and/or Pharmacology
Avutometinib
In repeat-dose toxicity studies up to 13-weeks duration in rats and monkeys, tissue mineralization in multiple organs was observed at doses ≥ 0.01 mg/kg/day and 0.03 mg/kg/day, respectively (≥ 0.06 and 1.4 times the human exposure at the recommended dose based on AUC, respectively) as well as increased inorganic phosphorus in rats.
In a cardiovascular telemetry study in monkeys, a single oral dose of avutometinib increased systolic, diastolic, and mean blood pressure at 1 mg/kg (approximately 15 times the human Cmax at the recommended dose). In the repeat-dose toxicology studies up to 13-weeks duration in rats, myocardial degeneration and necrosis were observed at doses ≥ 0.01 mg/kg/day (below the human exposure at the recommended dose based on AUC).
Defactinib
In a cardiovascular telemetry study in dogs, a single oral dose of defactinib decreased myocardial contractility at doses ≥ 5 mg/kg (approximately the human exposure at the recommended dose based on AUC) and increased ventricular systolic, diastolic and mean blood pressure at doses ≥ 25 mg/kg/day (≥ 2.7 times the human exposure at the recommended dose based on AUC). In the 13-week repeat-dose toxicology study in dogs, myocardium hypertrophy was observed at doses ≥ 1 mg/kg/day (below the human exposure at the recommended dose based on AUC) at the end of the dosing and recovery periods.
14. Clinical Studies
The efficacy of AVMAPKI FAKZYNJA CO-PACK was evaluated in RAMP-201 (NCT04625270), an open-label, multicenter study that included 57 adult patients with measurable KRAS-mutated recurrent LGSOC. Patients were required to have received at least one prior systemic therapy, including a platinum-based regimen. KRAS mutation status was determined by prospective local testing using next generation sequencing (NGS) or polymerase chain reaction of tumor tissue specimens. Patients were excluded if they were candidates for debulking surgery, were on treatment with warfarin, had an active skin disorder requiring systemic therapy within the past year, or had an ocular disorder (including a history of retinal pathology, an active or chronic visually significant corneal disorder, or a history of glaucoma).
Patients received AVMAPKI 3.2 mg orally twice weekly for the first 3 weeks out of a 4-week cycle and FAKZYNJA 200 mg orally twice daily for the first 3 weeks out of a 4-week cycle until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall response rate (ORR) assessed by blinded independent review committee (BIRC) according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. An additional efficacy outcome measure was duration of response (DoR). Tumor response assessments occurred every 8 weeks for the first 72 weeks and every 12 weeks thereafter.
The median age was 60 years (range: 29 to 87); 75% were White, 3.5% were Asian, 3.5% were Black or African American, and 18% did not have race reported; 3.5% of patients were Hispanic or Latino; 72% had an ECOG PS of 0 and 28% had ECOG PS of 1. The KRAS mutations identified by local testing were G12V (53%), G12D (35%), Q61H (3.5%), G12C (1.8%), G12R (1.8%), A146V (1.8%), and mutations not otherwise specified at G12x (1.8%) and on codon 12/13 (1.8%). Fourteen percent of patients had received 1 prior line of systemic therapy, 25% of patients had received 2 prior lines, 18% had received 3 prior lines and 40% had received more than 3 prior lines of systemic therapy. All patients had received prior platinum-based chemotherapy, 84% received prior hormonal therapy (as maintenance or treatment), 40% received prior bevacizumab and 21% received a prior MEK inhibitor.
Efficacy results are presented in Table 5.
| AVMAPKI FAKZYNJA CO-PACK N = 57 |
|
|---|---|
|
|
| Confirmed Overall Response Rate (95% CI)* | 44% (31,58) |
| Complete response | 3.5% |
| Partial response | 40% |
| Duration of Response (DoR) | |
| Range (months) | 3.3, 31.1 |
The tumor KRAS mutations observed in the 25 responders were A146V, G12D, G12R, G12V, and Q61H.
16. How is Amapki Fakzynja Co-Pack supplied
AVMAPKI FAKZYNJA CO-PACK is supplied in a carton that contains:
| AVMAPKI capsules in a 24-count bottle with child-resistant closure (NDC 71779-660-02) | NDC 71779-623-01 |
| FAKZYNJA tablets in a 42-count bottle with child-resistant closure (NDC 71779-630-01) |
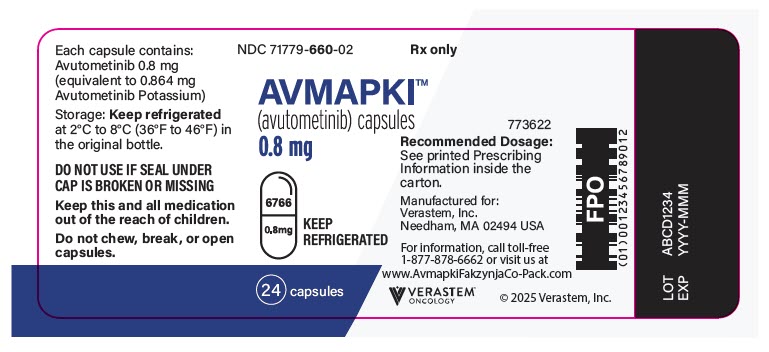
- AVMAPKI (avutometinib) 0.8 mg capsules are supplied as white capsules with "6766" printed on the cap and the strength "0.8 mg" printed on the body in black ink in a bottle containing 24 capsules. The bottle contains a desiccant that should not be discarded.
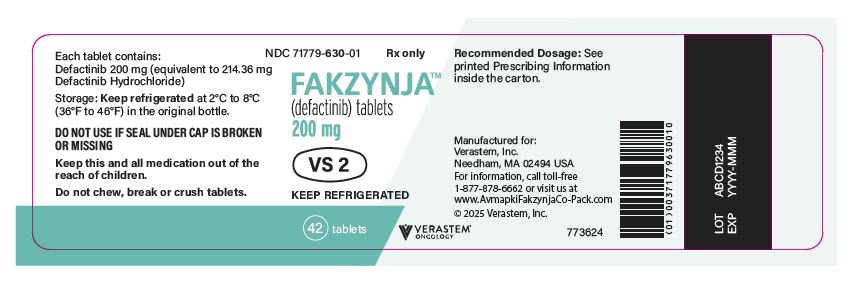
- FAKZYNJA (defactinib) 200 mg tablets are supplied as white to off-white, oval and debossed with "VS2" on one side of the tablet in a bottle containing 42 tablets.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Ocular Toxicities
Inform patients of the need for eye exams before and during treatment with AVMAPKI FAKZYNJA CO-PACK.
Advise patients to contact their healthcare provider if they experience any visual changes, pain, or inflammation around their eye(s) [see Warnings and Precautions (5.1)].
Serious Skin Toxicities
Inform patients that skin reactions have occurred during treatment with AVMAPKI FAKZYNJA CO-PACK.
Advise patients to use prophylactic topical steroids and oral antibiotics [see Dosage and Administration (2.2)] and to limit unnecessary exposure to sunlight with application of daily sunscreen (sun protection factor [SPF] ≥ 30) [see Warnings and Precautions (5.2)].
Advise patients to contact their healthcare provider if they develop a rash, progressively worsening skin reactions, or blistering of the skin or mouth [see Warnings and Precautions (5.2)].
Hepatotoxicity
Advise patients that they will need to undergo laboratory testing to monitor their liver function.
Advise patients to contact their healthcare provider for signs or symptoms of liver dysfunction [see Warnings and Precautions (5.3)].
Rhabdomyolysis
Inform patients that they will need to undergo laboratory testing to monitor CPK levels.
Advise patients to contact their healthcare provider for signs or symptoms of rhabdomyolysis [see Warnings and Precautions (5.4)].
Drug Interactions
Inform patients to avoid concomitant use of AVMAPKI FAKZYNJA CO-PACK with PPIs or H2 receptor antagonists.
Advise patients that if use of an acid reducing agent cannot be avoided, to take FAKZYNJA 2 hours prior to, or 2 hours after, the administration of a locally acting antacid [see Drug Interactions (7.1)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
Advise females to inform their healthcare providers of a known or suspected pregnancy. Advise females of reproductive potential to use effective contraception during treatment with AVMAPKI FAKZYNJA CO-PACK and for 1 month after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with AVMAPKI FAKZYNJA CO-PACK and for 4 months after the last dose [see Warnings and Precautions (5.5) and Use in Specific Population (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with AVMAPKI FAKZYNJA CO-PACK and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise males and females of reproductive potential that AVMAPKI FAKZYNJA CO-PACK may impair fertility [see Use in Specific Populations (8.3)].
Manufactured for and Distributed by:
Verastem, Inc.
Needham, Massachusetts 02494
1-877-878-6662
© 2025 Verastem, Inc.
| PATIENT INFORMATION AVMAPKI™ FAKZYNJA™ CO-PACK (ave-MAP-kee Fak-zin-jah koh-pak) (avutometinib capsules; defactinib tablets) co-packaged for oral use |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued: 05/2025 | ||||||||
| What is AVMAPKI FAKZYNJA CO-PACK?
AVMAPKI FAKZYNJA CO-PACK is a prescription medicine used to treat adults who have:
|
|||||||||
Before taking AVMAPKI FAKZYNJA CO-PACK, tell your healthcare provider about all of your medical conditions, including if you:
Especially tell your healthcare provider if you take:
How should I take AVMAPKI FAKZYNJA CO-PACK?
|
|||||||||
What should I avoid while taking AVMAPKI FAKZYNJA CO-PACK?
|
|||||||||
What are the possible side effects of AVMAPKI FAKZYNJA CO-PACK? AVMAPKI FAKZYNJA CO-PACK may cause serious side effects, including:
|
|||||||||
|
|
||||||||
|
|||||||||
|
|
|
|||||||
|
|||||||||
|
|
||||||||
|
|||||||||
|
|
|
|||||||
| The most common side effects of AVMAPKI FAKZYNJA CO-PACK include: | |||||||||
|
|
|
|
||||||
| AVMAPKI FAKZYNJA CO-PACK may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if this is a concern for you. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of AVMAPKI FAKZYNJA CO-PACK. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||||||
How should I store AVMAPKI FAKZYNJA CO-PACK?
|
|||||||||
| General information about the safe and effective use of AVMAPKI FAKZYNJA CO-PACK.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AVMAPKI FAKZYNJA CO-PACK for a condition for which it was not prescribed. Do not give AVMAPKI FAKZYNJA CO- PACK to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about AVMAPKI FAKZYNJA CO-PACK that is written for health professionals. |
|||||||||
| What are the ingredients in AVMAPKI FAKZYNJA CO-PACK?
AVMAPKI (avutometinib) capsules: Active ingredient: avutometinib Inactive ingredients: magnesium stearate, mannitol, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac, and strong ammonia solution. The capsule shell contains carrageenan, hypromellose, potassium chloride, purified water, and titanium oxide. FAKZYNJA (defactinib) tablets: Active ingredient: defactinib Inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose and sodium starch glycolate. Manufactured for and Distributed by: Verastem, Inc., Needham, Massachusetts 02494 © 2025 Verastem, Inc. For more information, go to www.AvmapkiFakzynjaCo-Pack.com or call 1-877-878-6662. |
|||||||||
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 71779-623-01
Rx only
AVMAPKI™
(avutometinib) capsules
0.8 mg
FAKZYNJA™
(defactinib) tablets
200 mg
CO-PACK
This carton contains:
AVMAPKI™ (avutometinib):
1 bottle of 24 capsules
FAKZYNJA™ (defactinib):
1 bottle of 42 tablets
Prescribing Information
Patient Dosing Card
KEEP REFRIGERATED
VERASTEM™
ONCOLOGY
PRINCIPAL DISPLAY PANEL - 0.8 mg Capsule Bottle Label
NDC 71779-660-02
Rx only
AVMAPKI™
(avutometinib) capsules
0.8 mg
KEEP
REFRIGERATED
24 capsules
773622
Recommended Dosage:
See printed Prescribing
Information inside the
carton.
Manufactured for:
Verastem, Inc.
Needham, MA 02494 USA
For information, call toll-free
1-877-878-6662 or visit us at
www.AvmapkiFakzynjaCo-Pack.com
VERASTEM™
ONCOLOGY
© 2025 Verastem, Inc.
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Label
NDC 71779-630-01
Rx only
FAKZYNJA™
(defactinib) tablets
200 mg
KEEP REFRIGERATED
42 tablets
VERASTEM™
ONCOLOGY
Recommended Dosage: See
printed Prescribing Information
inside the carton.
Manufactured for:
Verastem, Inc.
Needham, MA 02494 USA
For information, call toll-free
1-877-878-6662 or visit us at
www.AvmapkiFakzynjaCo-Pack.com
© 2025 Verastem, Inc.
773624
| AVMAPKI FAKZYNJA CO-PACK
avutometinib potassium and defactinib hydrochloride kit |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Verastem Inc. (032472566) |