8HR Arthritis Pain Relief
Dosage form: tablet, film coated, extended release
Ingredients: ACETAMINOPHEN 650mg
Labeler: CVS Pharmacy
NDC code: 69842-544
Medically reviewed by Drugs.com. Last updated on Sep 9, 2024.
Acetaminophen 650 mg
Pain reliever/fever reducer
- •
- temporarily relieves minor aches and pains due to:
- •
- minor pain of arthritis
- •
- muscular aches
- •
- backache
- •
- premenstrual and menstrual cramps
- •
- the common cold
- •
- headache
- •
- toothache
- •
- temporarily reduces fever
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 6 caplets in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
liver disease
taking the blood thinning drug warfarin
- •
- pain gets worse or lasts more than 10 days
- •
- fever gets worse or lasts more than 3 days
- •
- new symptoms occur
- •
- redness or swelling is present
These could be signs of a serious condition.
ask a health professional before use.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
- •
- do not take more than directed (see overdose warning)
|
adults |
|
|
under 18 years of age |
|
- •
- store at 20-25°C (68-77°F)
- •
- do not use if printed foil under cap is broken or missing
- •
- meets the requirements of USP Dissolution Test 2
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, stearic acid, titanium dioxide
1-800-719-9260
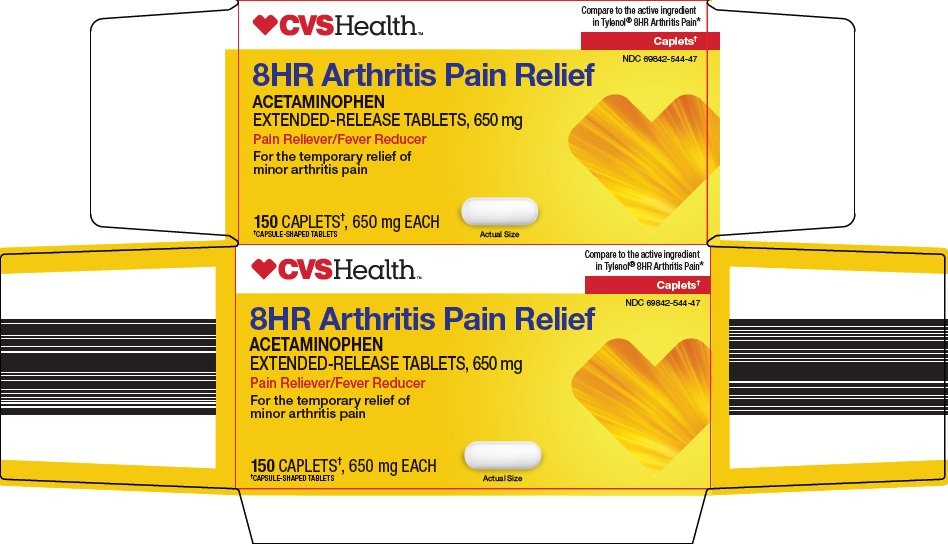
Compare to the active ingredient in Tylenol® 8HR Arthritis Pain
Caplets†
8HR Arthritis Pain Relief
ACETAMINOPHEN
EXTENDED-RELEASE TABLETS, 650 mg
Pain Reliever/Fever Reducer
For the temporary relief of minor arthritis pain
150 CAPLETS†, 650 mg EACH
†CAPSULE-SHAPED TABLETS
Actual Size

| 8HR ARTHRITIS PAIN RELIEF
acetaminophen tablet, film coated, extended release |
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
