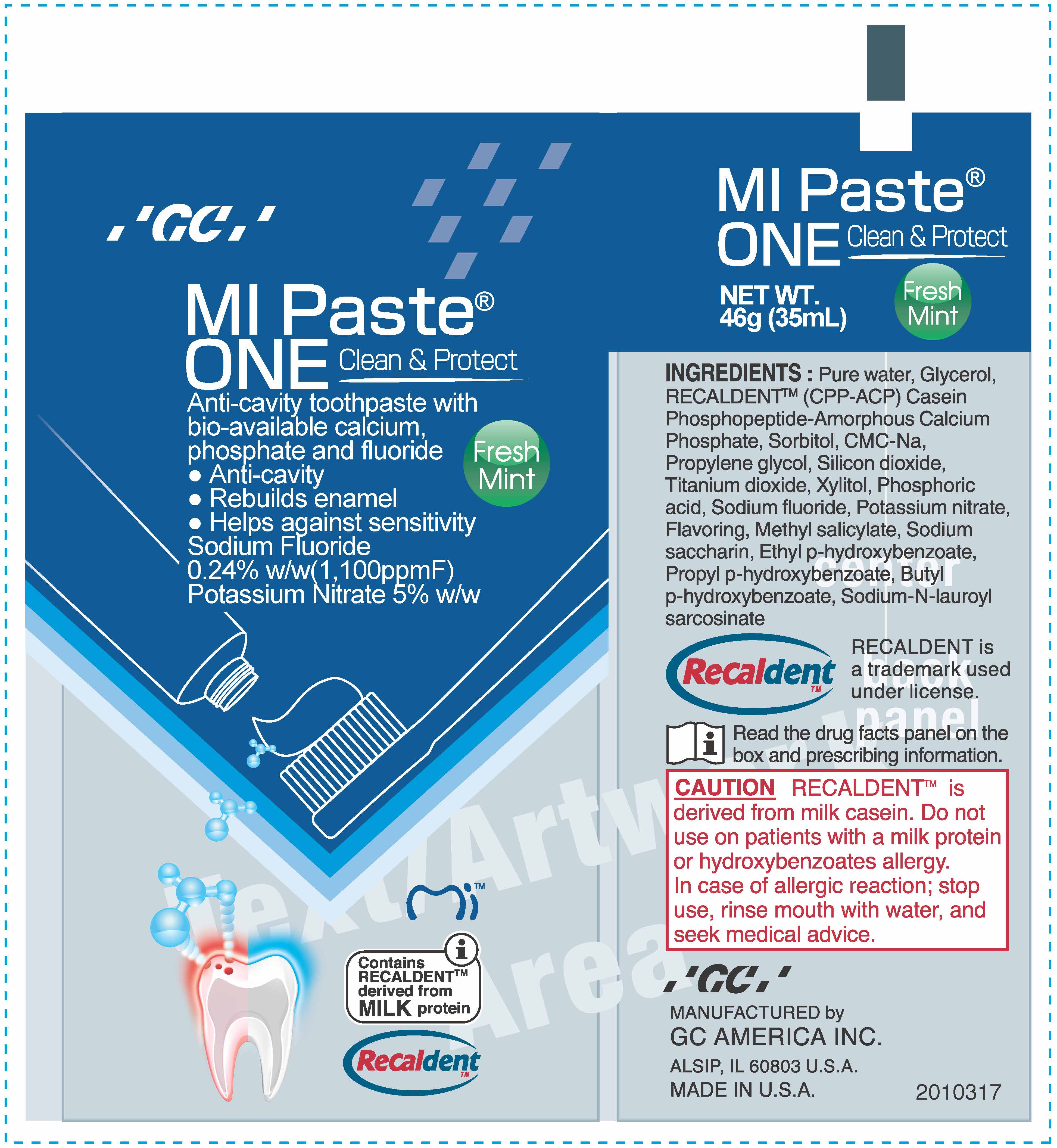
MIPaste ONE
Dosage form: paste, dentifrice
Ingredients: SODIUM FLUORIDE 0.0024g in 1g, POTASSIUM NITRATE 0.06g in 1g
Labeler: GC America Inc.
NDC code: 61596-437
Medically reviewed by Drugs.com. Last updated on Jan 10, 2025.
Sodium fluoride
0.24% w/w Anti-cavity
Potassium nitrate
5% (w/w)* Anti-sensitivity
*Maximum FDA sensitivity active ingredient
-aids in the prevention of dental cavities
-builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
Warning If the sensitivity persists after 4 weeks of use, consult a dentist or physician. Keep out of reach of children. If more than the recommended amount of paste used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. Do not use on patients with a milk protein and/or hydroxybenzoates allergy. In case of allergic reaction, stop use, rinse mouth with water and seek medical advice.
For adults and children 12 years of age or older, apply a small, pea-sized amount of MI Paste One to your toothbrush. For best results, brush for 2 minutes, expectorate and do not rinse; use twice a day. Replace cap after use. For children under 12 years of age, consult a dentist or physician.
Pure water, Glycerol, RECALDENT TM (CPP-ACP) Casein Phosphopeptide-Amorphous Calcium Phosphate, Sorbitol, CMC-Na, Propylene glycol, Silicon dioxide, Titanium dioxide, Xylitol, Phosphoric acid, flavoring, Methyl salicylate, Sodium saccharin, Ethyl p-hydroxybenzoate, Propyl p-hydroxybenzoate, Butyl p-hydroxybenzoate, Sodium-N-lauroyl sarcosinate
1-708-323-7063 www.gcamerica.com
Keep out of reach of children.
Anti-cavity toothpaste with bio-available calcium.
Anti-cavity
Rebuilds enamel
Helps against sensitivity
Sodium Fluoride 0.24% w/w (1,100ppmF)
Potassium Nitrate 5% w/w/
.jpg)
| MIPASTE
ONE
sodium fluoride, potassium nitrate paste, dentifrice |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - GC America Inc. (005473608) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GC America Inc. | 005473608 | manufacture(61596-437) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
