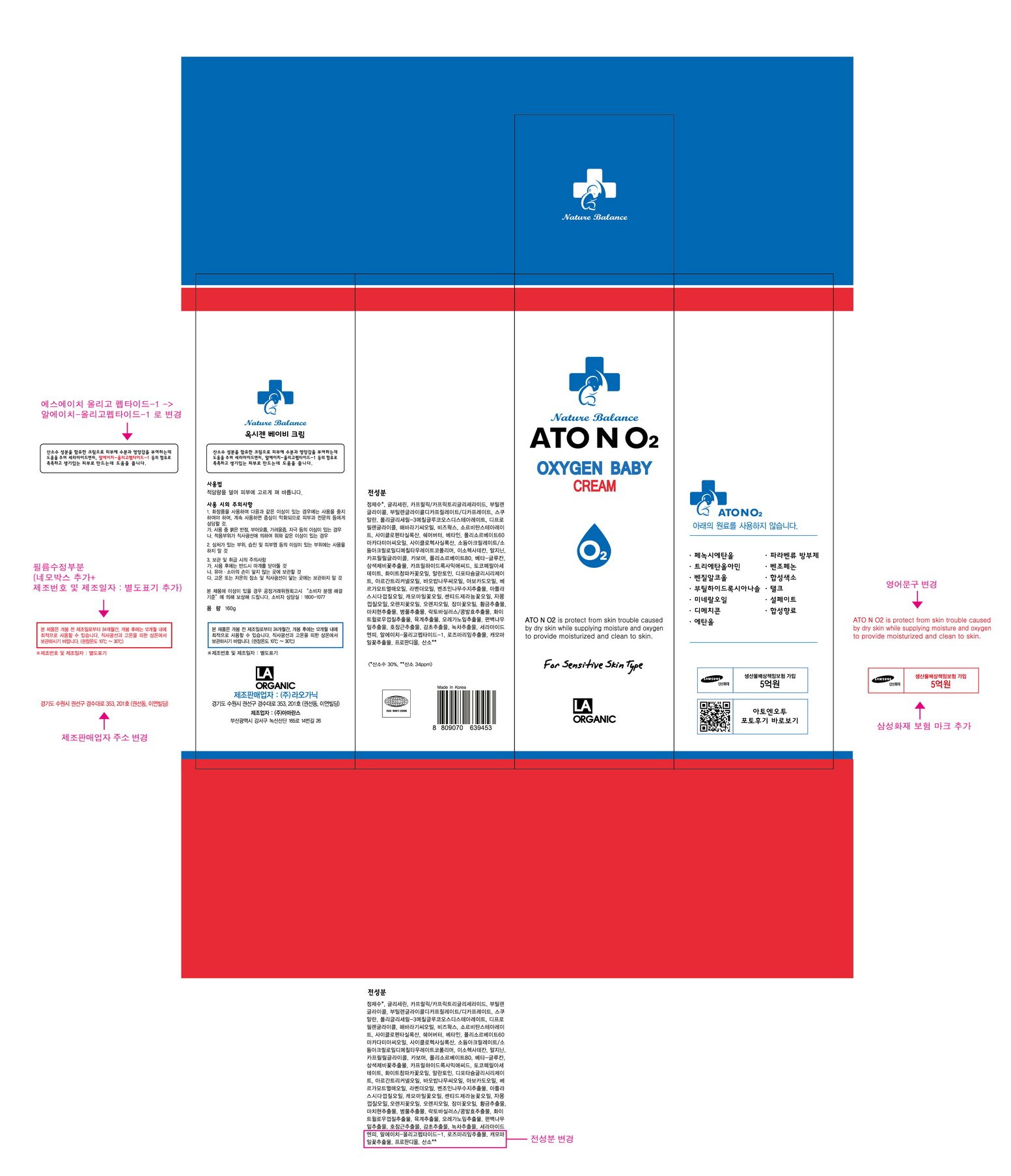
ATONO2 Oxygen Baby Cream
Dosage form: cream
Ingredients: GLYCERIN 8.06g in 100g
Labeler: LAORGANIC Co., Ltd.
NDC code: 70993-0011
Medically reviewed by Drugs.com. Last updated on Oct 3, 2024.
Glycerin
Water, Caprylic/Capric Triglyceride, Butylene Glycol, Butylene Glycol Dicaprylate/Dicaprate, Polyglyceryl-3 Methylglucose Distearate, Squalane, Dipropylene Glycol, Helianthus Annuus (Sunflower) Seed Oil, Beeswax, Cyclopentasiloxane, Sorbitan Stearate, Butyrospermum Parkii (Shea) Butter, Macadamia Integrifolia Seed Oil, Betaine, etc.
Skin Protectant
keep out of reach of the children
Apply proper amount to the skin.
- For external use only
- When using this product
1. If any one of following symptoms occurs when using cosmetics, stop using it and consult a dermatologist.
A. red spot, swelling, itching, stimulus
B. symptoms mentioned above are caused by sunlight.
2. Do not apply on skin where there is wound, eczema, or irritation.
3. Storage and cautions for handling
A. Close stopper after using.
B. Keep out of reach of children.
C. Keep away from high or low temperature and direct sunlight.
4. This is not a medicinal product. Made of 100% cosmetic ingredients, it is a sensitive-skin care product for all skin types.
for external use only
| ATONO2 OXYGEN BABY CREAM
glycerin cream |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - LAORGANIC Co., Ltd. (689844148) |
| Registrant - LAORGANIC Co., Ltd. (689844148) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LAORGANIC Co., Ltd. | 689844148 | manufacture(70993-0011) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
