Provon Antimicrobial Foam Handwash
Dosage form: solution
Ingredients: CHLORHEXIDINE GLUCONATE 20mg in 1mL
Labeler: Gojo Industries, Inc.
NDC code: 21749-850
Medically reviewed by Drugs.com. Last updated on Jun 9, 2025.
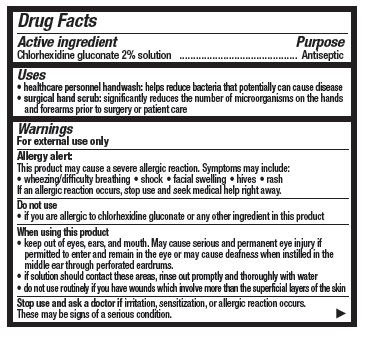
Drug Facts
Chlorhexidine gluconate 2% solution
Antiseptic
- healthcare personnel handwash: helps reduce bacteria that potentially can cause disease
- surgical hand scrub: significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
For external use only
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away
- if you are allergic to chlorhexidine gluconate or any other ingredient in this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums
- if solution should contact these areas, rinse out promptly and thoroughly with water
- do not use routineely if you have wounds which involve more than the superficial layers of the skin
Stop use and ask a doctor if irritation, sensitization or allergic reaction occurs. These may be signs of a serious condition.
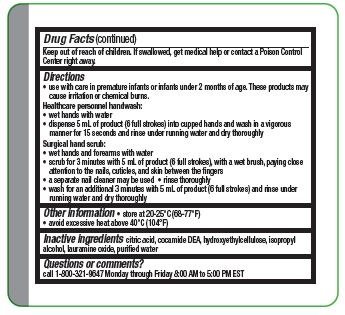
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- wet hands and forearms with water
- scrub for 3 minutes with 5 ml of product (6 full strokes), with a wet brush, paying close attention to the nails, cuticles, and skin between the fingers
- a separate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 mL of product (6 full strokes) and rinse under running water and dry thoroughly
- wet hands with water
- dispense 5 ml of product (6 full strokes) into cupped hands and wash in a vigorous manner for 15 seconds and rinse under running water and dry thoroughly
- store at 20-25° C (68-77° F)
- avoid excessive heat above 40° C (104° F)
citric add. cocamide DEA, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, purified water
Questions or comments?
call 1-800-321-9647 Monday through Friday 8 AM to 5 PM
PROVON® Antimicrobial Foam Handwash
Chlorhexidine Gluconate 2% Solution - Antiseptic
GOJOADXFLBLC
8842-643-F

PROVON® Antimicrobial Foam Handwash
Chlorhexidine Gluconate 2% Solution - Antiseptic
GOJOLTXFLBLC
1922-643-F

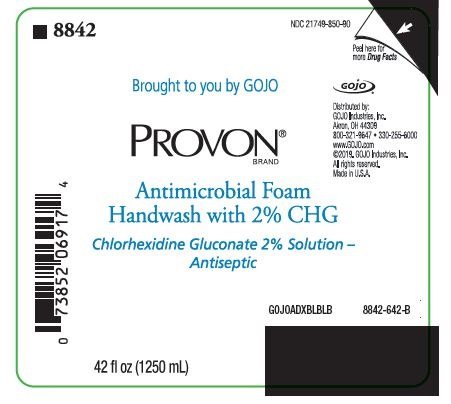
NDC 21749-850-90
Brought to you by GOJO
PROVON® BRAND
Antimicrobial Foam Handwash with 2% CHG
Chlorhexidine Gluconate 2% Solution - Antiseptic
Distributed by:
GOJO Industries, Inc.
Akron, OH 44309
800-321-9647 • 330-255-6000
www.GOJO.com
©2019. GOJO Industries, Inc.
All rights reserved.
Made in U.S.A.
GOJOADXBLBLB
8842-642-B
42 fl oz (1250 mL)

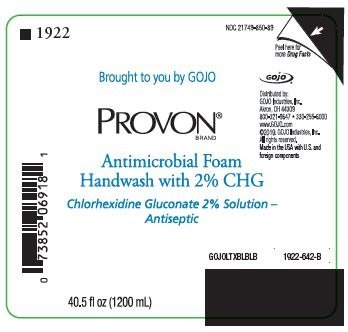
NDC 21749-850-89
Brought to you by GOJO
PROVON® BRAND
Antimicrobial Foam Handwash with 2% CHG
Chlorhexidine Gluconate 2% Solution - Antiseptic
Distributed by:
GOJO Industries, Inc.
Akron, OH 44309
800-321-9647 • 330-255-6000
www.GOJO.com
©2019. GOJO Industries, Inc.
All rights reserved.
Made in the USA with U.S. and foreign components
GOJOLTXBLBLB
1922-642-B
40.5 fl oz (1200 mL)

| PROVON ANTIMICROBIAL FOAM HANDWASH
chlorhexidine gluconate 2% solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Gojo Industries, Inc. (004162038) |
| Registrant - Xttrium Laboratories, Inc. (007470579) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
