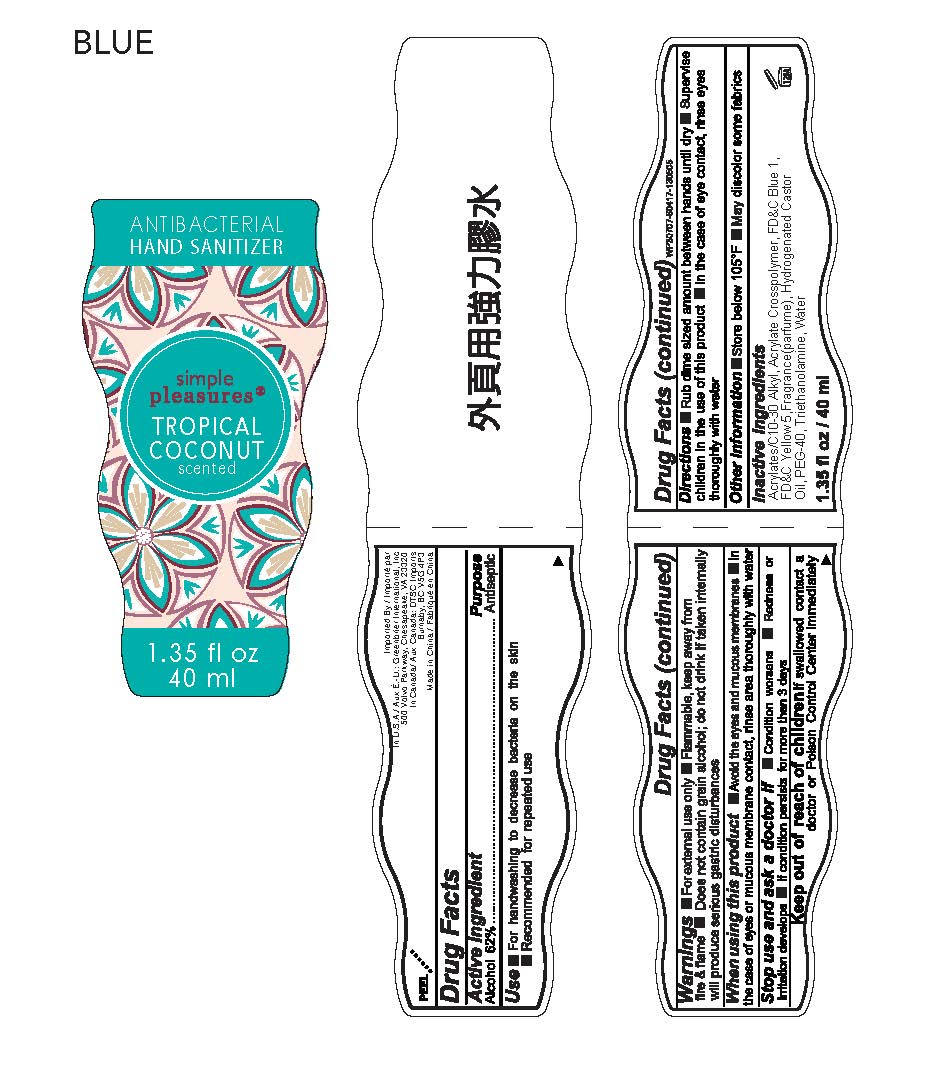
simple pleasures ANTIBACTERIAL HAND SANITIZER
Dosage form: liquid
Ingredients: ALCOHOL 62g in 100mL
Labeler: GREENBRIER INTERNATIONAL, INC.
NDC code: 33992-1419
Medically reviewed by Drugs.com. Last updated on Nov 26, 2024.
Alcohol 62%
Antiseptic
■ For handwashing to decrease bacteria on the skin
■ Recommended for repeated use
■ For external use only ■ Flammable, keep away from fire & flame ■ Does not contain grain alcohol;do not drink. If taken internally will produce serious gastric disturbances
■ Avoid the eyes and mucous membranes ■ In the case of eyes or mucous membrane contact, rinse area thoroughly with water
■ Condition worsens ■ Redness or irritation develops ■ If condition persists for more than 3 days
If swallowed contact a doctor or Poison Control Center immediately.
■ Rub dime sized amount between hands until dry ■ Supervise children in the use of this product ■ In the case of eye contact, rinse eyes thoroughly with water
■ Store below 105°F ■ May discolor some fabrics
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, FD&C Blue 1, FD&C Yellow 5, Fragrance (parfume), Hydrogenated Castor Oil, PEG-40, Triethanolamine, Water
| SIMPLE PLEASURES
ANTIBACTERIAL HAND SANITIZER
ethyl alcohol liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - GREENBRIER INTERNATIONAL, INC. (610322518) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
