Aerosoothe
Dosage form: ointment
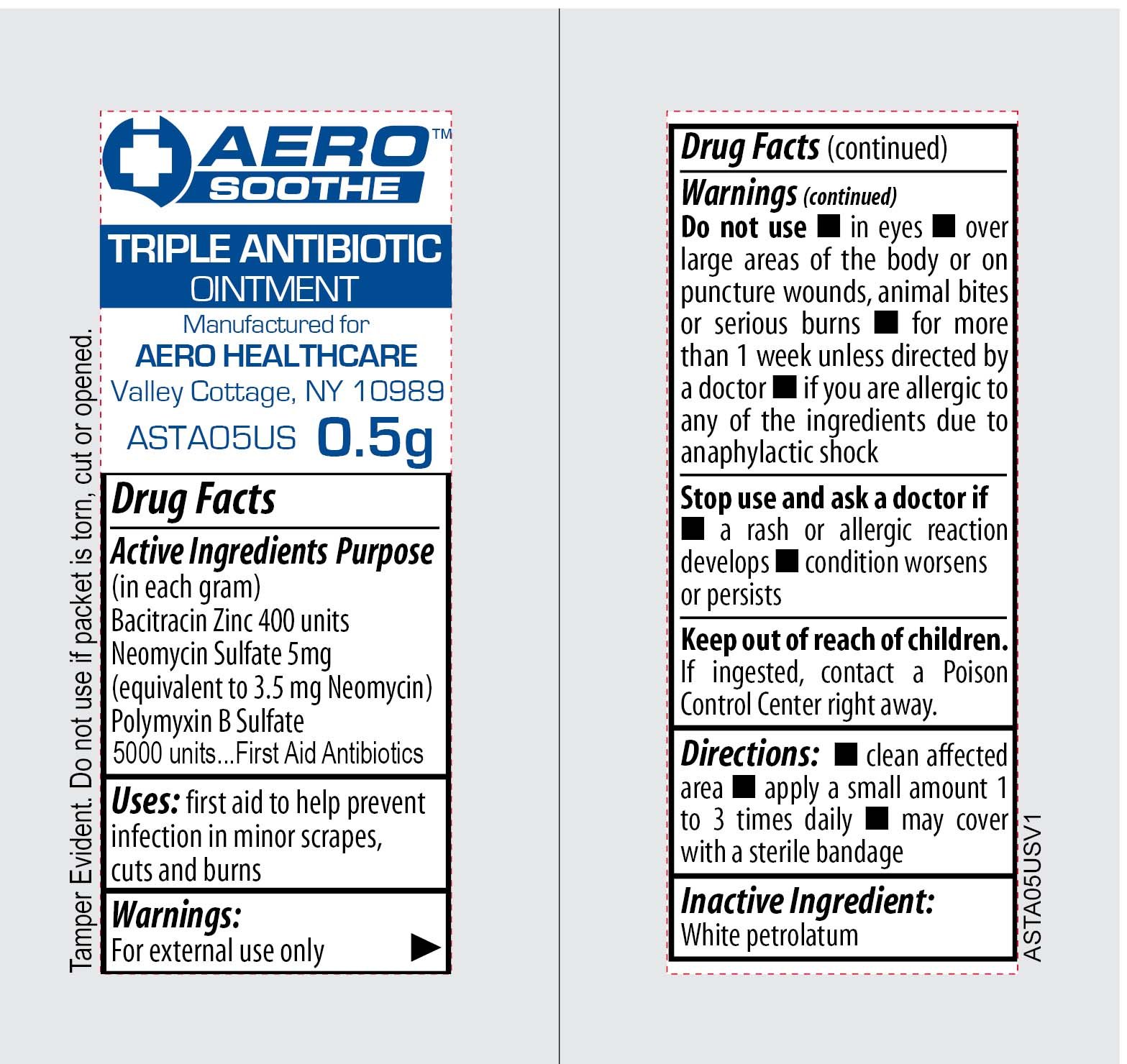
Ingredients: POLYMYXIN B SULFATE 5000[iU] in 1g, BACITRACIN ZINC 400[iU] in 1g, NEOMYCIN SULFATE 5mg in 1g
Labeler: Aero Healthcare US LLC
NDC code: 55305-123
Medically reviewed by Drugs.com. Last updated on Sep 2, 2025.
ACTIVE INGREDIENT
Active Ingredients: In each gram-Neomycin Sulfate-5 mg (equivalent to 3.5 mg Neomycin), Polymixin B Sulfate-5000 I.U., Bacitracin Zinc-400 I.U.
PURPOSE
WARNINGS
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN.
IF INGESTED, CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
- CLEAN AFFECTED AREA
- APPPLY A SMALL AMOUNT 1 TO 3 TIMES DAILY
- MAY COVER WITH A STERILE BANDAGE
INACTIVE INGREDIENT
INACTIVE INGREDIENT: WHITE PETROLATUM
Indications & Usage
FIRST AID TO HELP PREVENT INFECTION IN MINOR SCRAPES, CUTS AND BURNS.
AST05USV1
| AEROSOOTHE
triple antibiotic ointment ointment |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Aero Healthcare US LLC (008186174) |
Document Id: 58ffb850-d58f-0dc0-e053-2a91aa0a1015
Set id: 2287a31c-750c-728d-e054-00144ff88e88
Version: 2
Aero Healthcare US LLC
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
