Boraqua Eye Wash
Dosage form: solution
Ingredients: WATER 99.05mL in 100mL
Labeler: Genuine Drugs
NDC code: 69666-871
Medically reviewed by Drugs.com. Last updated on Jul 7, 2025.
Purpose
Eyewash
Active ingredient
Purified water (99.05%)
Uses
washes the eye to help relieve
- irritation
- stinging
- discomfort
- itching;
by removing
- loose foreign material
- air pollutants (smog or pollen)
- chlorinated water
Warnings
Do not use
- if you have open wounds in or near the eyes, and get medical help right away
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- condition worsens or persists
Directions
Remove contact lenses before using.
- Do not touch tip of container to any surface to avoid contamination.
- Replace cap after use.

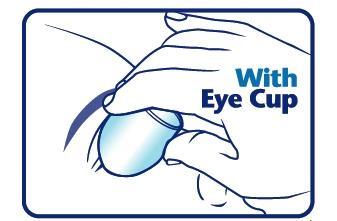
When using an eye cup
- rinse the cup with Pure-aid Eye Wash immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with Pure-aid Eye Wash Solution and apply the cup to the affected eye(s), pressing tightly to prevent spillage
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye
- rinse cup with clean water after each use
Other information
- store at room temperature
- keep tightly closed
- use before expiration date marked on the carton or bottle
Inactive ingredients
boric acid, sodium borate, sodium chloride, Hydrochloric acid PRESERVATIVE ADDED: edetate disodium, polyhexamethylene biguanide
Boraqua

| BORAQUA EYE WASH
water solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Genuine Drugs (079610378) |
Document Id: aa846bb7-a64f-3358-e053-2995a90a9d09
Set id: 142c742e-4860-7392-e054-00144ff88e88
Version: 4
Genuine Drugs
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
