CurX Antimicrobial Gel
Dosage form: gel
Ingredients: BENZALKONIUM CHLORIDE 0.13g in 100g
Labeler: Global Health Solutions, LLC
NDC code: 69578-466
Medically reviewed by Drugs.com. Last updated on Oct 18, 2024.
Benzalkonium Ch. 0.13% (i/s*)
First Aid Antiseptic
first aid to to help treat, protect and prevent skin infection associated with
- minor cuts
- scrapes
- burns
For external use only
- deep or puncture wounds
- animal bites
- serious burns
- Do not use in or near the eyes
- do not use if you have a history of allergy to any of the ingredients.
- condition worsens
- symptoms persist for more than 7 days, or clear up and occur again within a few days.
If swallowed, get medical help or contact a Poison Control Center right away.
- adults and children 2 years and older: clean the affected area; apply a small amount on the area 1-3 times daily;may be covered with a sterile bandage (let dry first)
- children under 2 years: ask a doctor
Avoid excessive heat
USP Petrolatum, Water, Polihexanide (PHMB)
1-706-844-1025
GLOBAL HEALTH SOLUTIONS, LLC
CurXTM Antimicrobial Gel
First Aid Antimicrobial Gel
Hospital and Professional Use Only
Non-Antibiotic
5 g
CurX is a registered trademark of Global Health Solutions
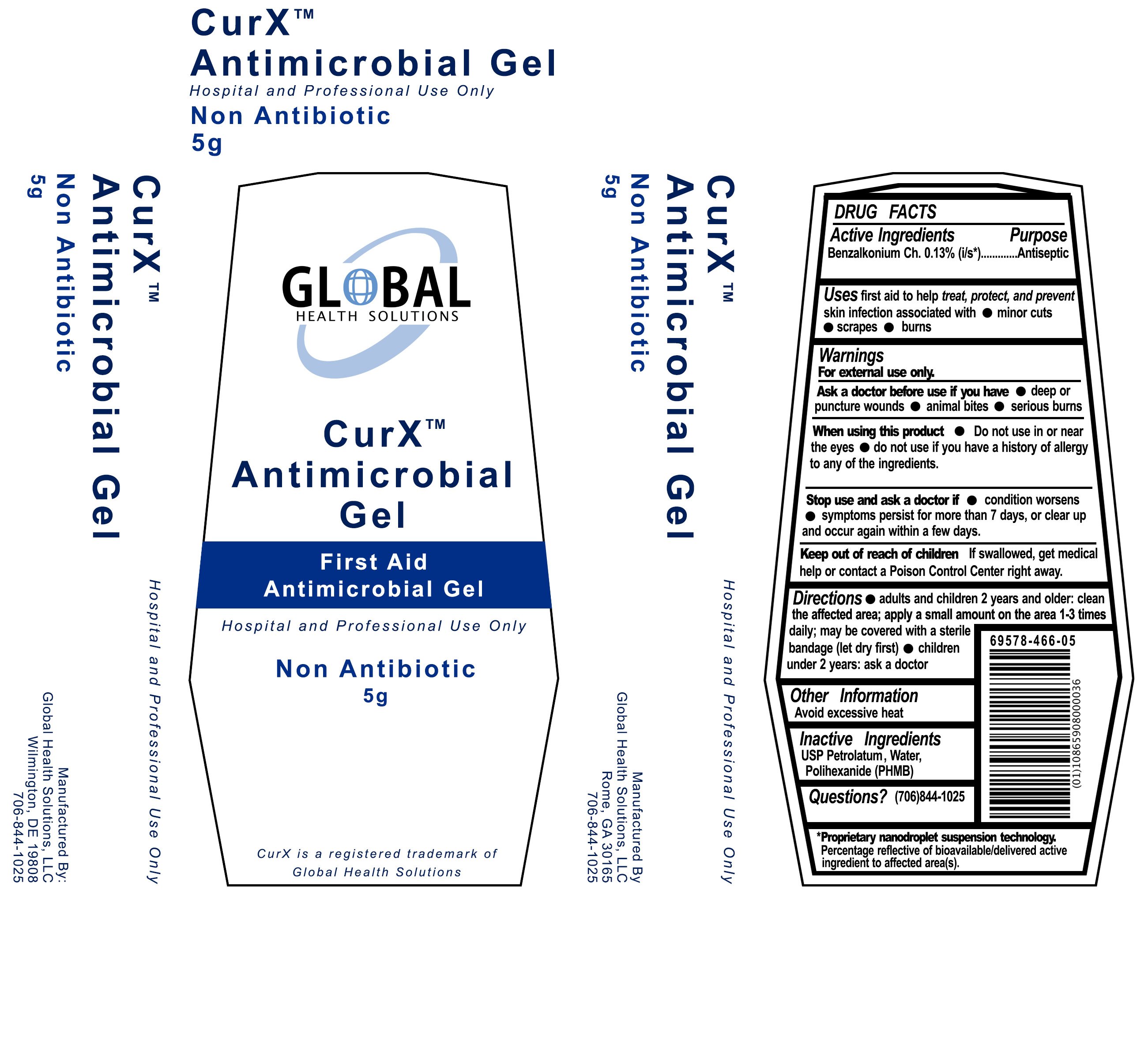
*Proprietary microdroplet suspension technology. Percentage reflective of active ingredient bioavailability in solution.
| CURX ANTIMICROBIAL GEL
benzalkonium chloride gel |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - Global Health Solutions, LLC (079695890) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Global Health Solutions, LLC | 079695890 | manufacture(69578-466) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
