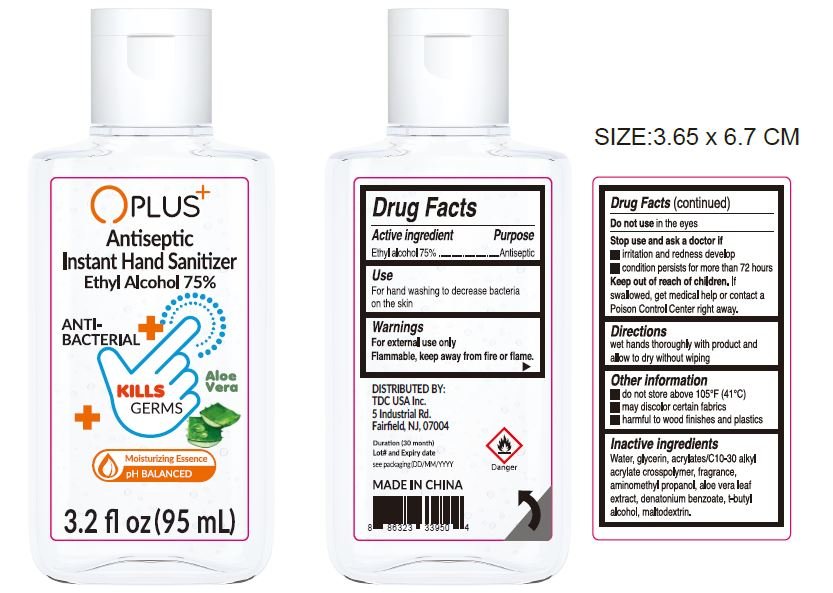
OPLUS Antiseptic Instant Hand Sanitizer
Dosage form: gel
Ingredients: ALCOHOL 0.75mL in 1mL
Labeler: TDC USA Inc
NDC code: 79204-000
Medically reviewed by Drugs.com. Last updated on Jun 27, 2025.
Drug Facts
Active ingredient
Ethyl alcohol 75%
Purpose
Antiseptic
Use
For hand washing to decrease bacteria on the skin
Warnings
For external use only
Flammable, keep away from fire or flame.
Do not use
in the eyes
Stop use and ask a doctor if
- irritation and redness develop
- condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
wet hands thoroughly with product and allow to dry without wiping
Other information
- do not store above 105°F (41°C)
- may discolor certain fabrics
- harmful to wood finishes and plastics
Inactive ingredients
Water, glycerin, acrylates/C10-30 alkyl acrylate crosspolymer, fragrance, aminomethyl propanol, Aloe vera leaf extract, denatonium benzoate, t-butyl alcohol, maltodextrin
Package Labeling
| OPLUS ANTISEPTIC INSTANT HAND SANITIZER
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - TDC USA Inc (054204290) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Opal Cosmetics (Huizhou) Limited | 528178475 | manufacture(79204-000) | |
Document Id: a9da7642-4b00-1fb4-e053-2a95a90af0a3
Set id: df15a4ae-c420-4cae-940a-7a75b884afa2
Version: 3
TDC USA Inc
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
