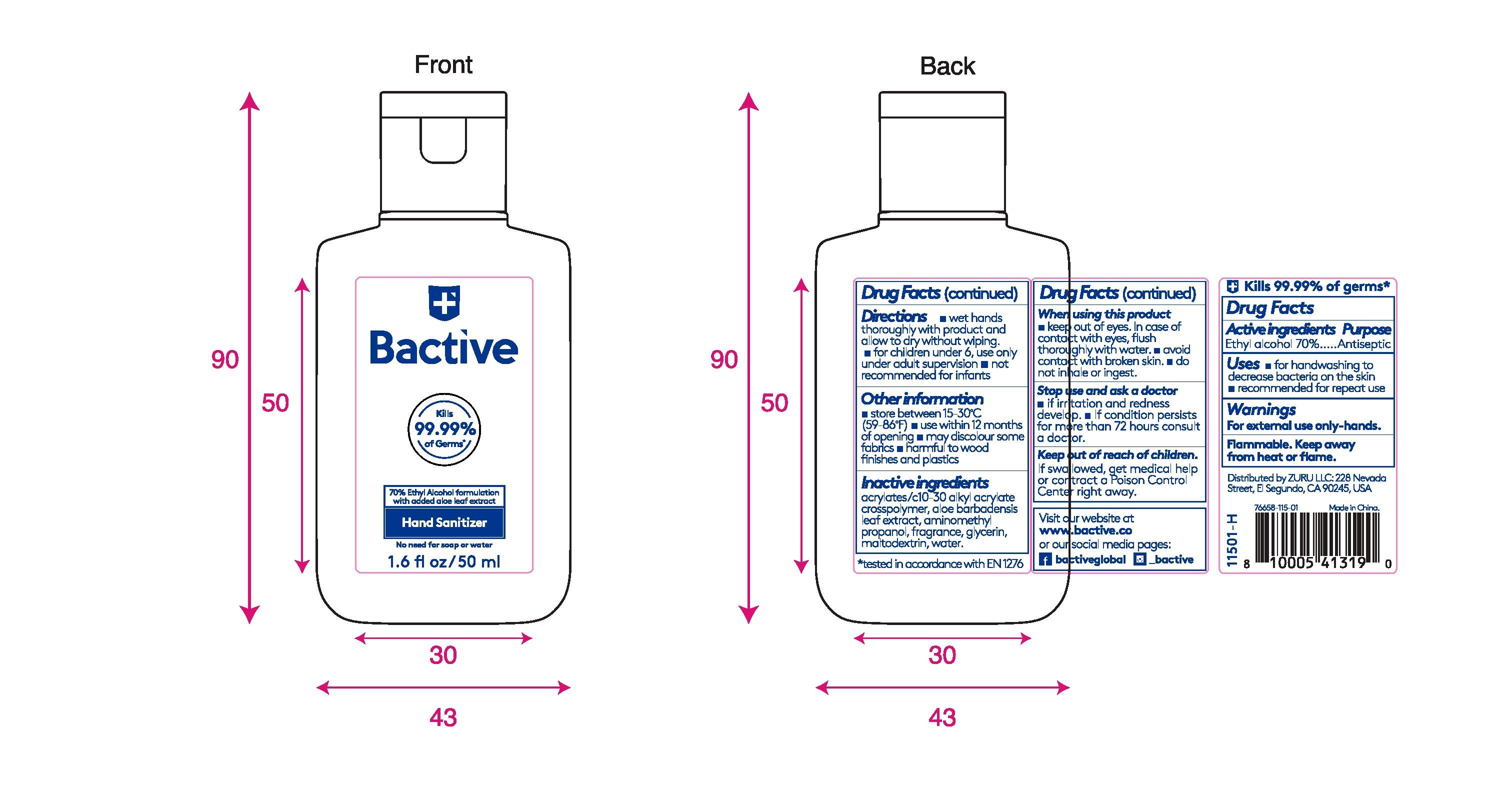
Bactive Hand Sanitizer
Dosage form: gel
Ingredients: ALCOHOL 70mL in 100mL
Labeler: Zuru LLC
NDC code: 76658-115
Medically reviewed by Drugs.com. Last updated on Jun 6, 2025.
Ethyl Alcohol 70%.
Antiseptic
- For handwashing to decrease bacteria on the skin
- Recommended for repeat use
For external use only - hands. Flammable. Keep away from heat or flame.
When using this product
■ keep out of eyes. In case of contact with eyes, flush thoroughly with water.
■ avoid contact with broken skin.
■ do not inhale or ingest.
Stop use and ask a doctor
- If irritation and redness develop.
- If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- wet hands thoroughly with product and allow to dry without wiping
- for children under 6, use only under adult supervision
- not recommended for infants
- Store between 15-30C (59-86F)
- use within 12 months of opening
- may discolour some fabrics
- harmful to wood finishes and plastics
acrylates/c10-30 alkyl acrylate crosspolymer, aloe barbedensis leaf extract, aminomethyl propanol, fragrance, glycerin, maltodextrin, water
50 mL
500 mL

2L

| BACTIVE HAND SANITIZER
ethyl alcohol gel |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Zuru LLC (080435986) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Opal Cosmetics (Huizhou) Limited | 528178475 | manufacture(76658-115), label(76658-115) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
