GSD Good Hygiene Defense Wet Wipes
Dosage form: cloth
Ingredients: BENZALKONIUM CHLORIDE 1mg in 1g
Labeler: JoCo Sales & Marketing, Inc.
NDC code: 77784-001
Medically reviewed by Drugs.com. Last updated on Aug 21, 2025.
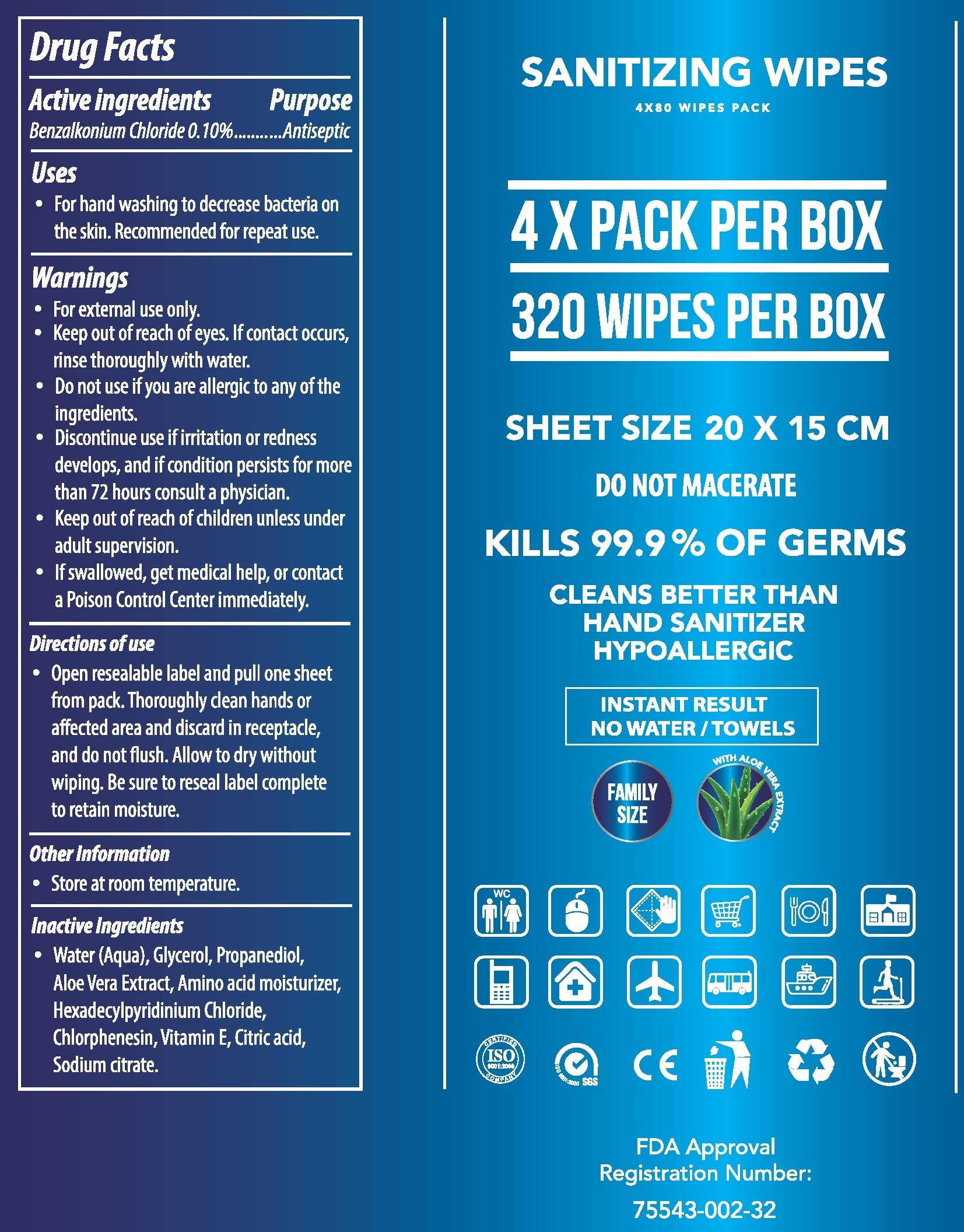
Drug Facts
Active ingredients
Benzalkonium Chloride 0.10%
Purpose
Antiseptic
Uses
- For hand washing to decrease bacteria on the skin. Recommended for repeat use.
Warnings
- For external use only.
- Keep out of reach of eyes. If contact occurs, rinse thoroughly with water.
Do not use
- if you are allergic to any of the ingredients.
- Discontinue use if irritation or redness develops, and if condition persists for more than 72 hours consult a physician.
Keep out of reach of children
- unless under adult supervision.
- If swallowed, get medical help, or contact a Poison Control Center immediately.
Directions of use
- Open resealable label and pull one sheet from pack. Thoroughly clean hands or affected area and discard in receptacle, and do not flush. Allow to dry without wiping. Be sure to reseal label complete to retain moisture.
Other Information
- Store at room temperature.
Inactive Ingredients
- Water (Aqua), Glycerol, Propanediol, Aloe Vera Extract, Amino acid moisturizer, Hexadecylpyridinium Chloride, Chlorphenesin, Vitamin E, Citric acid, Sodium citrate.
Package Labeling:20ct

Package Labeling:80ct

Package Labeling: 77784-001-21, 200ct


77784-001-32 package label
80 wipes in a bag, 4 bags in a box. 320 Wipes total.
80 wipes in a bag, 4 bags in a box. 320 Wipes total.

800ct Package label


| GSD GOOD HYGIENE DEFENSE WET WIPES
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - JoCo Sales & Marketing, Inc. (157016291) |
Document Id: ae2eb310-89be-4d21-e053-2995a90a72bd
Set id: 4df1c19e-07d7-4afd-acfd-5a9c031e599e
Version: 4
JoCo Sales & Marketing, Inc.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
