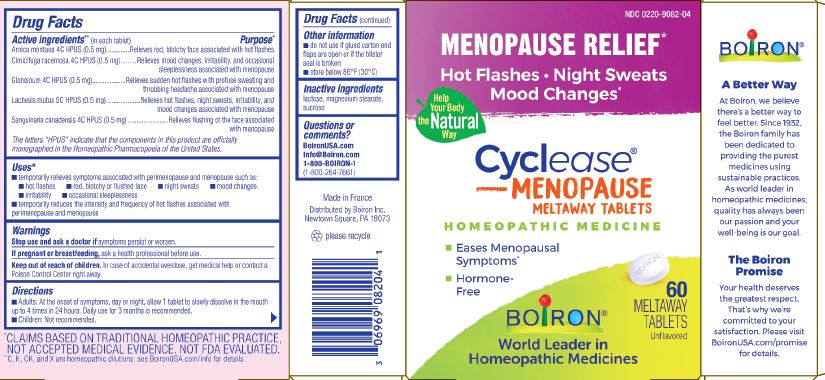
Cyclease Menopause
Dosage form: tablet
Ingredients: BLACK COHOSH 4[hp_C], LACHESIS MUTA VENOM 5[hp_C], SANGUINARIA CANADENSIS ROOT 4[hp_C], NITROGLYCERIN 4[hp_C], ARNICA MONTANA WHOLE 4[hp_C]
Labeler: Boiron
NDC code: 0220-9082
Medically reviewed by Drugs.com. Last updated on Mar 21, 2025.
Arnica montana 4C HPUS (0.5mg)
Cimicifuga racemosa 4C HPUS (0.5mg)
Glonoinum 4C HPUS (0.5mg)
Lachesis mutus 5C HPUS (0.5mg)
Sanguinaria canadensis 4C HPUS (0.5mg)
Arnica montana 4C HPUS (0.5mg) ... Relieves red, blotchy face associated with hot flashes
Cimicifuga racemosa 4C HPUS (0.5mg) ... Relieves mood changes, irritability, and occassional sleeplessness associated with menopause.
Glonoinum 4C HPUS (0.5mg) ... Relieves sudden hot flashes with profuse sweating and throbbing headache associated with menopause
Lachesis mutus 5C HPUS (0.5mg) ... Relieves hot flashes, nigh sweats, irrability, and mood changes associated with menopause
Sanguinaria canadensis 4C HPUS (0.5mg) ... Relieves flushing of the face associated with menopause
temporarily relieves symptoms associated with perimenopause and menopause such as: hot flashes, red, blotchy or flushed face, night sweats, mood changes, irritability, occasional sleeplessness, temporarily reduces the intensity and frequency of hot flashes associated with perimenopause and menopause
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Adults: At the onset of symptoms, day or night, allow 1 tablet to slowly dissolve in the mouth up to 4 times in 24 hours. Daily use for 3 months is recommended. Children: Not recommended.
Do not use if glued carton end flaps are open or if the blister seal is broken.
Store below 86° F (30° C)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
60 meltaway tablets
Menapause relief*
lactose, magnesium stearate, sucrose
1-800-BOIRON-1 (1-800-264-7661),
BoironUSA.com Info@boiron.com
Distributed by Boiron, Inc. Newtown Square, PA 19073
Made in France
| CYCLEASE MENOPAUSE
arnica montana whole, black cohosh, nitroglycerin, lachesis muta venom, sanguinaria canadensis root tablet |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Boiron (282560473) |
| Registrant - Boiron Inc. (014892269) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Boiron | 282560473 | manufacture(0220-9082) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
