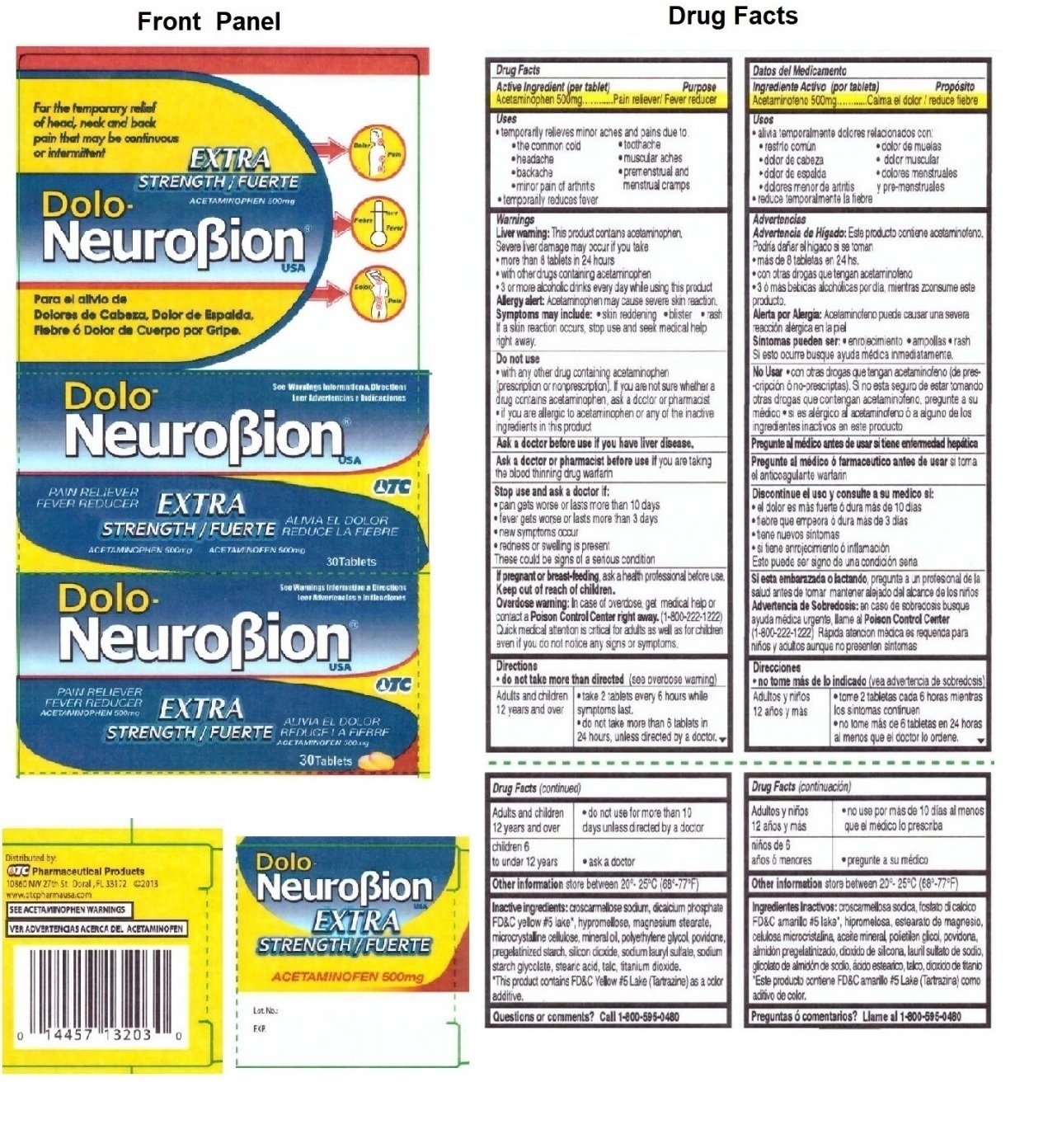
DOLO- NeuroBion Acetaminophen 500mg
Dosage form: tablet, film coated
Ingredients: ACETAMINOPHEN 500mg
Labeler: BENARD INDUSTRIES INC
NDC code: 55959-171
Medically reviewed by Drugs.com. Last updated on Oct 29, 2024.
Acetaminophen 500mg
Pain reliever/ Fever reducer
• temporarily relieves minor aches and pains due to:
• the common cold • toothache
• headache • muscular aches
• backache • premenstrual and menstrual cramps
• minor pain of arthritis
• temporarily reduces fever
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if you take
• more than 8 tablets in 24 hours
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reaction.
Symptoms may include: • skin reddening • blister • rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
• with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
• if you are allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor before use if you have liver disease.
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
Stop use and ask a doctor if:
• pain gets worse or lasts more than 10 days
• fever gets worse or lasts more than 3 days
• new symptoms occur
• redness or swelling is present
These could be signs of a serious condition
If pregnant or breast-feeding, ask a health professional before use.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
• do not take more than directed (see overdose warning)
| Adults and children 12 years and over |
• take 2 tablets every 6 hours while symptoms last. • do not take more than 6 tablets in 24 hours, unless directed by a doctor. • do not use for more than 10 days unless directed by a doctor |
| children 6 to under 12 years |
• ask a doctor |
store between 20°- 25°C (68°-77°F)
croscarmellose sodium, dicalcium phosphate, FD&C yellow #5 lake*, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil, polyethylene glycol, povidone, pregelatinized starch, silicon dioxide, sodium lauryl sulfate, sodium starch glycolate, stearic acid, talc, titanium dioxide.
*This product contains FD&C yellow #5 Lake (Tartrazine) as a color additive.
Call 1-800-595-0480
For the temporary relief of head, neck and back pain that may be continuous or intermittent
EXTRA STRENGTH
See Warnings Information & Directions
PAIN RELIEVER
FEVER REDUCER
Distributed by:
OTC Pharmaceutical Products
10860 NW 27th St. Doral, FL 33172 ©2013
www.otcpharmausa.com
SEE ACETAMINOPHEN WARNINGS

| DOLO- NEUROBION
ACETAMINOPHEN 500MG
acetaminophen tablet, film coated |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - BENARD INDUSTRIES INC (106700321) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
