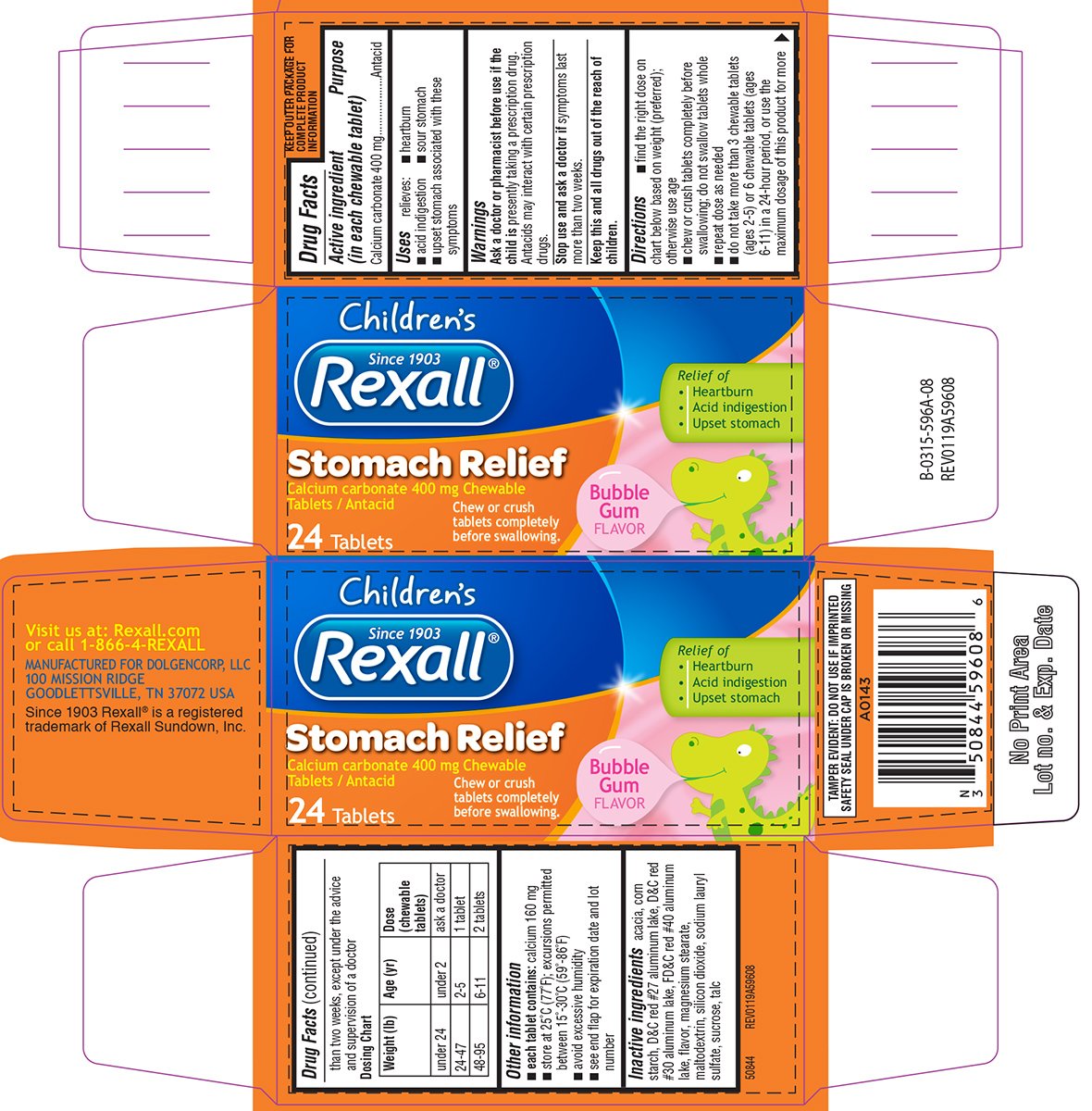
Stomach Relief Childrens
Dosage form: tablet, chewable
Ingredients: CALCIUM CARBONATE 400mg
Labeler: DOLGENCORP, LLC
NDC code: 55910-596
Medically reviewed by Drugs.com. Last updated on Sep 3, 2025.
Calcium carbonate 400 mg
Antacid
relieves:
- heartburn
- acid indigestion
- sour stomach
- upset stomach associated with these symptoms
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
symptoms last more than two weeks.
- find the right dose on chart below based on weight (preferred); otherwise use age
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose as needed
- do not take more than 3 chewable tablets (ages 2-5) or 6 chewable tablets (ages 6-11) in a 24-hour period, or use the maximum dosage of this product for more than two weeks, except under the advice and supervision of a doctor
Dosing Chart
| Weight (lb) | Age (yr) | Dose (chewable tablets) |
| under 24 | under 2 | ask a doctor |
| 24-47 | 2-5 | 1 tablet |
| 48-95 | 6-11 | 2 tablets |
- each tablet contains: calcium 160 mg
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- avoid excessive humidity
- see end flap for expiration date and lot number
acacia, corn starch, D&C red #27 aluminum lake, D&C red #30 aluminum lake, FD&C red #40 aluminum lake, flavor, magnesium stearate, maltodextrin, silicon dioxide, sodium lauryl sulfate, sucrose, talc
Children's
Since 1903
Rexall®
Stomach Relief
Calcium carbonate 400 mg chewable Tablets / Antacid
24 Tablets
Relief of
• Heartburn
• Acid indigestion
• Upset stomach
Bubble Gum Flavor
Chew or crush tablets completely before swallowing.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Visit us at: Rexall.com
or call 1-866-4-REXALL
MANUFACTURED FOR DOLGENCORP, LLC
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072 USA
Since 1903 Rexall® is a registered
trademark of Rexall Sundown, Inc.
50844 REV0119A59608
Rexall 44-596A
| STOMACH RELIEF
CHILDRENS
calcium carbonate tablet, chewable |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - DOLGENCORP, LLC (068331990) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK(55910-596) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE(55910-596) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 967626305 | PACK(55910-596) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867837 | PACK(55910-596) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 868734088 | PACK(55910-596) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
