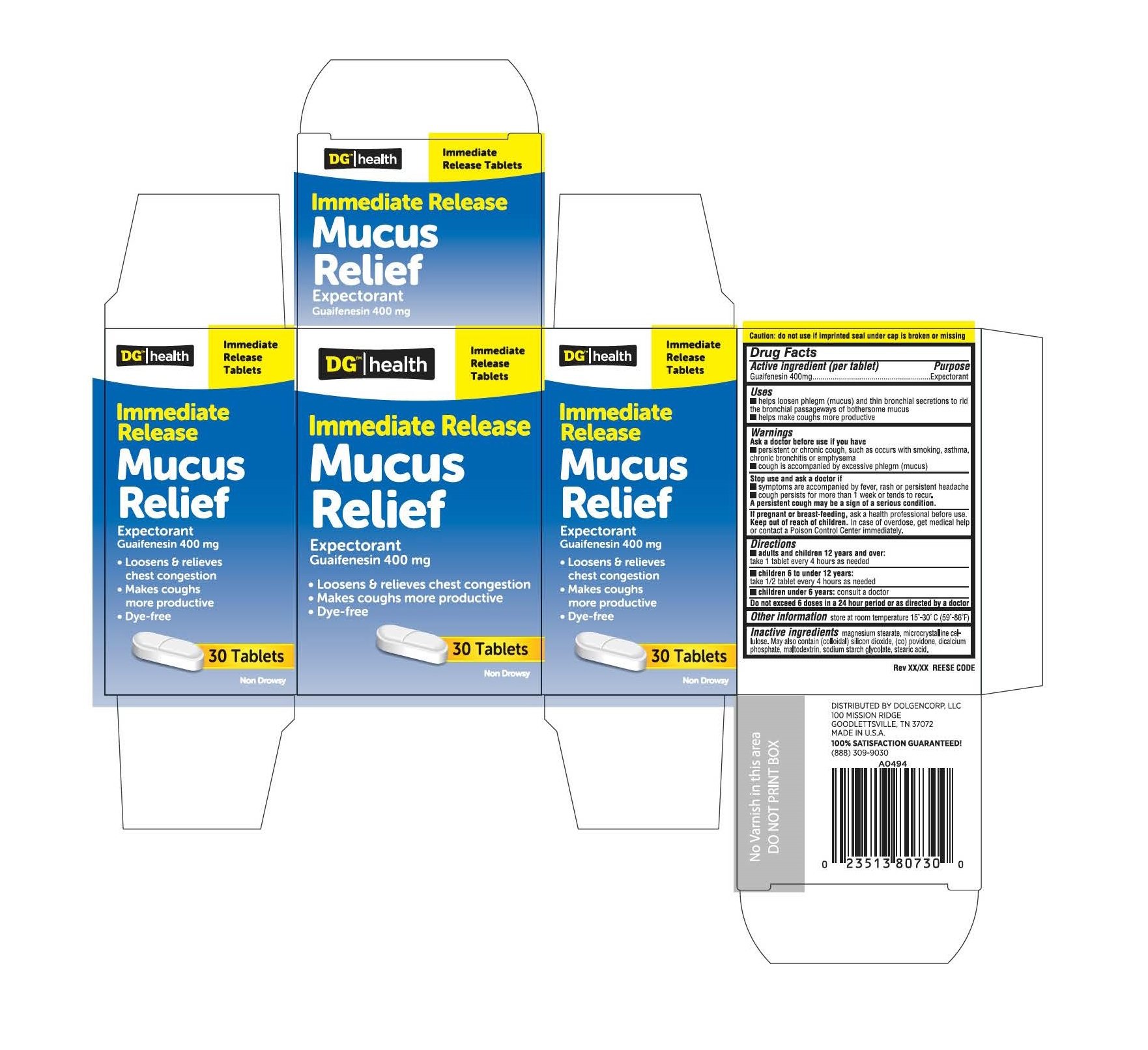
DG Health Immediate Release Mucus Relief
Dosage form: tablet
Ingredients: Guaifenesin 400mg
Labeler: Reese Pharmaceutical Co
NDC code: 10956-059
Medically reviewed by Drugs.com. Last updated on Jun 23, 2025.
Active ingredient (per tablet)
Guaifenesin 400mg
Expectorant
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus
- helps make coughs more productive
Ask doctor before use if you have
- persistent or chronic cough, such as occurs with smoking, asthma, bronchitis or emphysema
- cough is accompanied by excessive phlegm (mucus)
Stop use and ask doctor if
- Symptoms are accompanied by fever, rash or persistent headache
- cough persists for more than 1 week or tends to recur
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control
Center immediately.
- Adults and children 12 years of age and over: take 1 tablet every 4 hours as needed
- Children 6 to 10 under 12 years of age: take 1/2 tablet every 4 hours as needed
- Children under 6 years of age: consult a doctor
store at 15°-30°C (59°-86°F)
magnesium stearate, microcrystalline cellulose. May also contain (colloidal) silicon dioxide, (co) povidone, dicalcium phosphate, maltodextrin, sodium starch glycolate, stearic acid.
| DG HEALTH IMMEDIATE RELEASE MUCUS RELIEF
guaifenesin tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Reese Pharmaceutical Co (004172052) |
| Registrant - Reese Pharmaceutical Co (004172052) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Reese Pharmaceutical Co | 004172052 | relabel(10956-059), repack(10956-059) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pharbest | 557054835 | manufacture(10956-059) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
