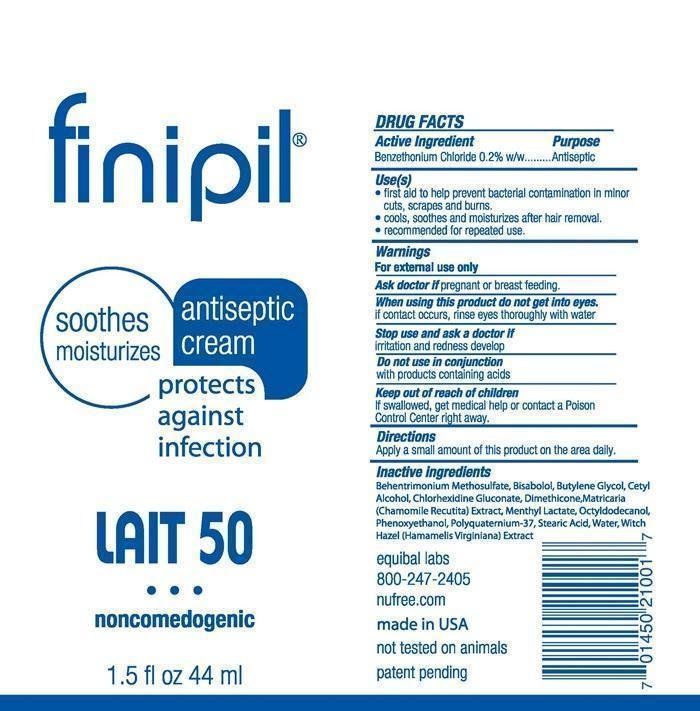
finipil Lait 50
Dosage form: cream
Ingredients: BENZETHONIUM CHLORIDE 195mg in 100mL
Labeler: Equibal, Inc.
NDC code: 53228-050
Medically reviewed by Drugs.com. Last updated on Oct 21, 2024.
DRUG FACTS
Active Ingredient
Benzethonium Chloride 0.2% w/w
Purpose
Antiseptic
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Use(s)
- first aid to help prevent bacterial contamination in minor cuts, scrapes and burns.
- cools, soothes and moisturizes after hair removal.
- recommended for repeated use.
Warnings
For external use only
Do not use in conjunction
with products containing acids
Ask a doctor if pregnant or breast feeding,
Stop use and ask a doctor if
irritation and redness develop
Directions
Apply a small amount of this product on the area daily.
When using this product do not get into eyes.
If contact occurs, rinse eyes thoroughly with water.
Inactive ingredients
Behentrimonium methosulfate, Bisabolol, Butylene Glycol, Cetyl Alcohol, Chlorhexidine Gluconate, Dimethicone, Matricaria (Chamomile Recutita) Extract, Menthyl Lactate, Octyldodecanol, Phenoxyethanol, Polyquarternium-37, Stearic Acid, Witch Hazel (Hamamelis Virginiana) Extract
finipil ®
soothes moisturizes
antiseptic cream
protects against infection
LAIT 50
noncomedogenic
1.5 fl oz 44 ml
equibal labs
800-247-2405
nufree.com
made in U.S.A.
not tested on animals
patent pending

| FINIPIL LAIT 50
benzethonium chloride cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Equibal, Inc. (144679883) |
| Registrant - Equibal, Inc. (144679883) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Equibal, Inc. | 144679883 | manufacture(53228-050) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
