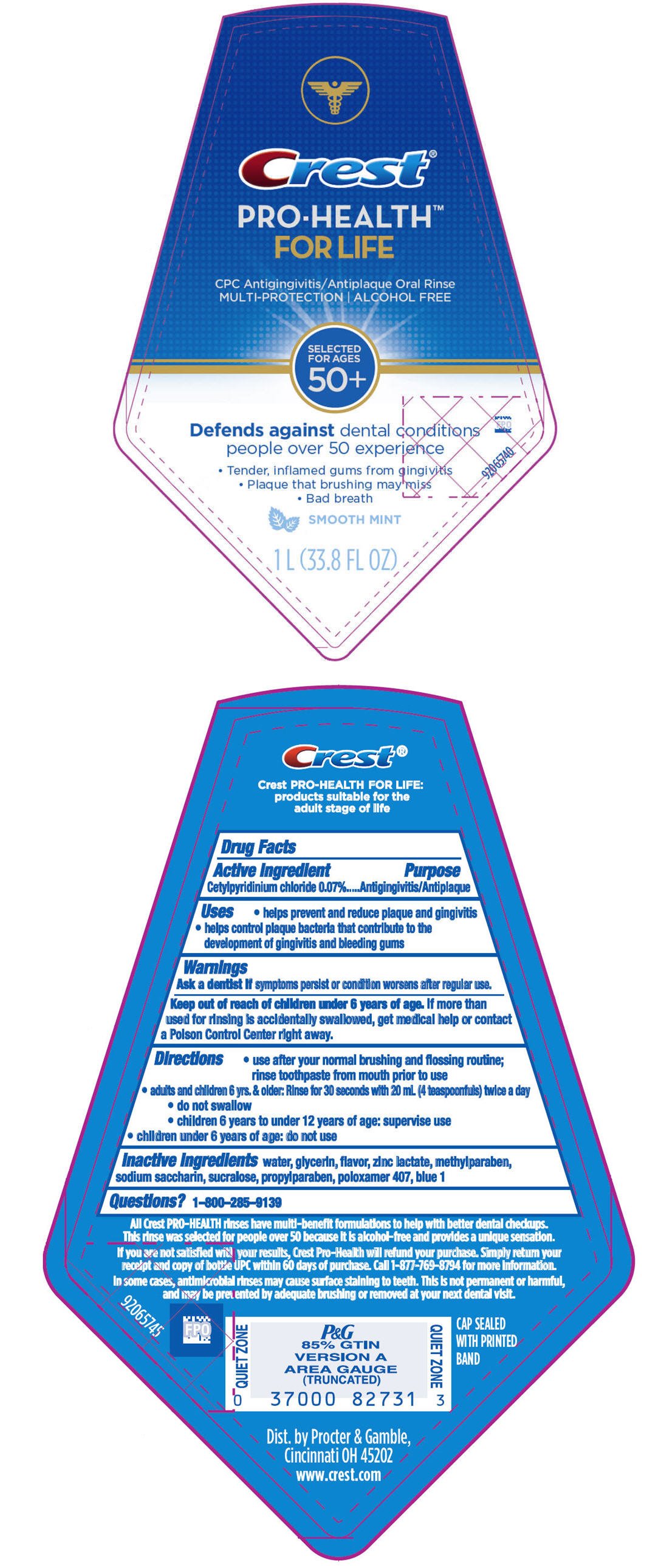
Crest Pro-Health For Life
Dosage form: rinse
Ingredients: CETYLPYRIDINIUM CHLORIDE 0.014g in 20mL
Labeler: The Procter & Gamble Manufacturing Company
NDC code: 37000-836
Medically reviewed by Drugs.com. Last updated on Feb 20, 2025.
For Life
Drug Facts
Cetylpyridinium chloride 0.07%
Antigingivitis/Antiplaque
- helps prevent and reduce plaque and gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis and bleeding gums
Ask a dentist if symptoms persist or condition worsens after regular use.
Keep out of reach of children under 6 years of age. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
- use after your normal brushing and flossing routine; rinse toothpaste from mouth prior to use
- adults and children 6 yrs. & older: Rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- children under 6 years of age: do not use
water, glycerin, flavor, zinc lactate, methylparaben, sodium saccharin, sucralose, propylparaben, poloxamer 407, blue 1
1-800-285-9139
Dist. by Procter & Gamble,
Cincinnati OH 45202
Crest®
PRO-HEALTH™
FOR LIFE
CPC Antigingivitis/Antiplaque Oral Rinse
MULTI-PROTECTION | ALCOHOL FREE
SELECTED
FOR AGES
50+
Defends against dental conditions
people over 50 experience
- Tender, inflamed gums from gingivitis
- Plaque that brushing may miss
- Bad breath
SMOOTH MINT
1 L (33.8 FL OZ)
92065740
| CREST PRO-HEALTH
FOR LIFE
cetylpyridinium chloride rinse |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
