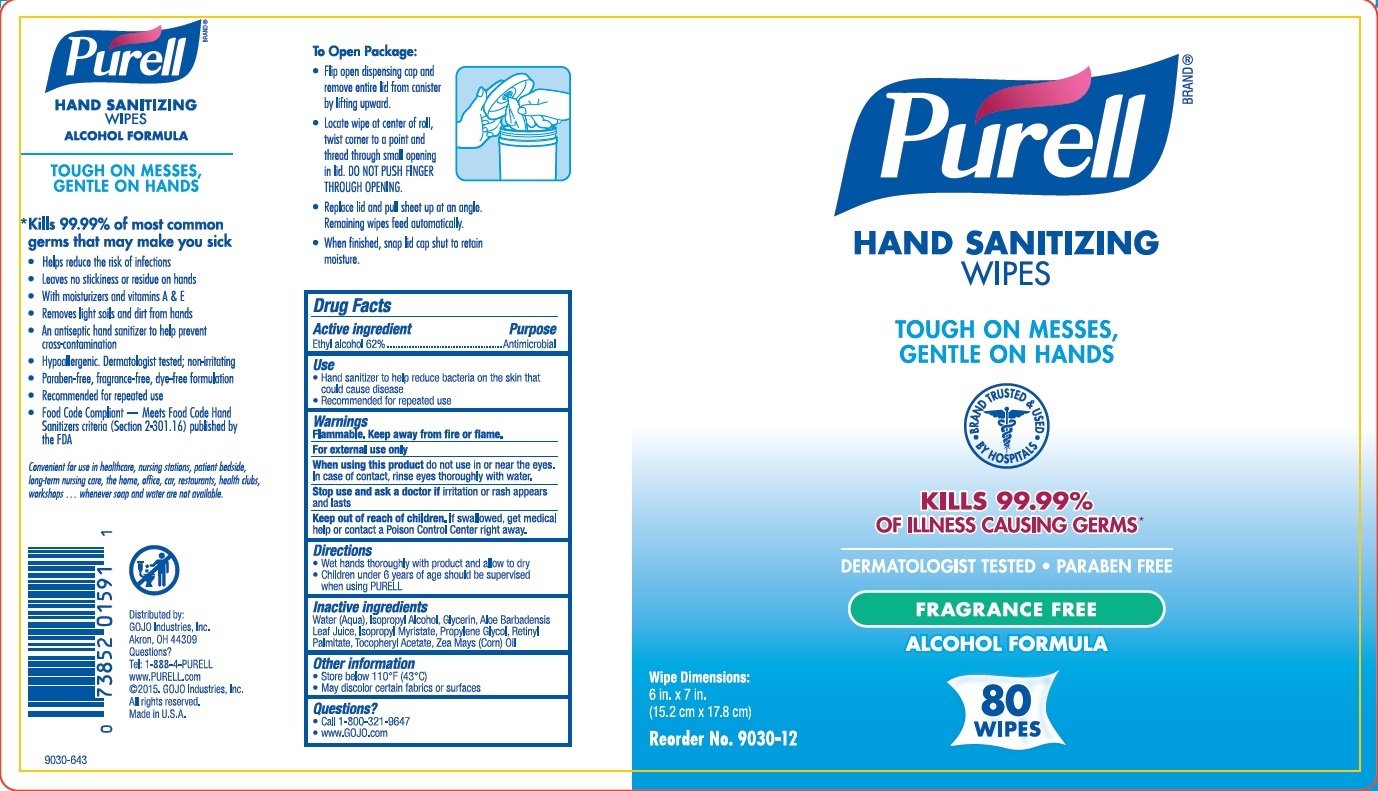
PURELL Hand Sanitizing Wipes Alcohol Formula
Dosage form: cloth
Ingredients: ALCOHOL 62mL in 100mL
Labeler: GOJO Industries, Inc.
NDC code: 21749-367
Medically reviewed by Drugs.com. Last updated on Aug 15, 2024.
Ethyl alcohol 62%
Antimicrobial
Hand sanitizer to help reduce bacteria on the skin
Flammable. Keep away from fire or flame. For external use only
When using this product do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash appears and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
• Wet hands thoroughly with product and allow to dry
• Children under 6 years of age should be supervised when using PURELL® products
• Store below 110°F (43°C)
• May discolor certain fabrics or surfaces
Water (Aqua), Isopropyl Alcohol, Glycerin, Aloe Barbadensis Leaf Juice, Isopropyl Myristate, Propylene Glycol, Retinyl Palmitate, Tocopheryl Acetate, Zea Mays (Corn) Oil
| PURELL HAND SANITIZING WIPES ALCOHOL FORMULA
alcohol cloth |
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| Labeler - GOJO Industries, Inc. (004162038) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GOJO Industries, Inc. | 036424534 | manufacture(21749-367) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
See also:
Qulipta
Qulipta is used to help prevent episodic or chronic migraine headaches in adults. Qulipta is an ...
Aimovig
Learn about Aimovig (erenumab-aooe) a once-monthly, injectable medication that can be ...
Dupixent
Dupixent is used to treat eczema, eosinophilic or oral-corticosteroid-dependent asthma, chronic ...
Ubrelvy
Ubrelvy (ubrogepant) tablets are used for the acute treatment of migraine. Includes Ubrelvy side ...
Nurtec ODT
Nurtec ODT (rimegepant) is used to treat acute migraines and prevent episodic migraines, by ...
Xeomin
Xeomin (incobotulinumtoxinA) is used to treat cervical dystonia, blepharospasm, upper facial lines ...
Dysport
Dysport (abobotulinumtoxinA) is used to treat cervical dystonia, glabellar lines and limb ...
Botox Cosmetic
Botox Cosmetic is a prescription treatment for fine lines and wrinkles. It temporarily improves the ...