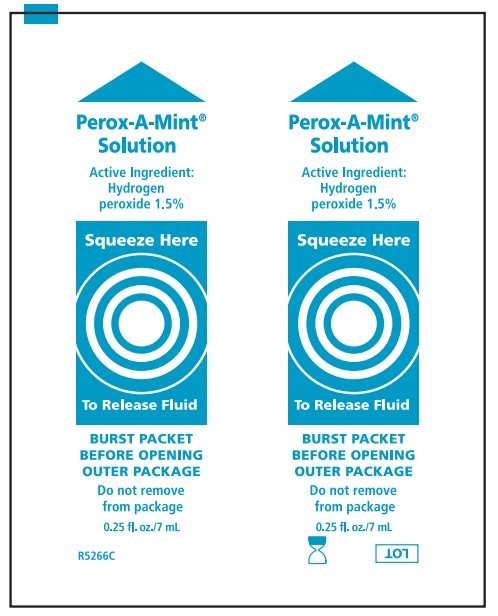
Perox-A-Mint Solution
Dosage form: mouthwash
Ingredients: HYDROGEN PEROXIDE 15mg in 1mL
Labeler: Sage Products, LLC
NDC code: 53462-075
Medically reviewed by Drugs.com. Last updated on Apr 10, 2025.
Hydrogen Peroxide 1.5%
Oral Debriding Agent
Aids in the removal of secretions associated with sore mouth
Do not use:
- For more than 7 days unless told to do so by a doctor.
- Avoid swallowing.
Stop use and ask a doctor if:
- Sore mouth symptoms do not improve in 7 days.
- Swelling, rash or fever develops.
- Irritation, pain or redness persists of worsens.
Keep out of reach of children. If more than used for debriding is accidentally swallowed, get medical help or contact a Poison Control Center right away.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Adults and children 3 years and over: rinse mouth for approx. one minute with 1/2 Tbsp. Instruct to expectorate.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
Water, menthol flavor, polysorbate 80, phosphoric acid, sodium saccharin, Blue 1 (CI42090), Yellow 6 (CI15985)
Questions? Call toll free 800-323-2220

| PEROX-A-MINT SOLUTION
hydrogen peroxide mouthwash |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Sage Products, LLC (054326178) |
| Registrant - Sage Products, LLC (054326178) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
