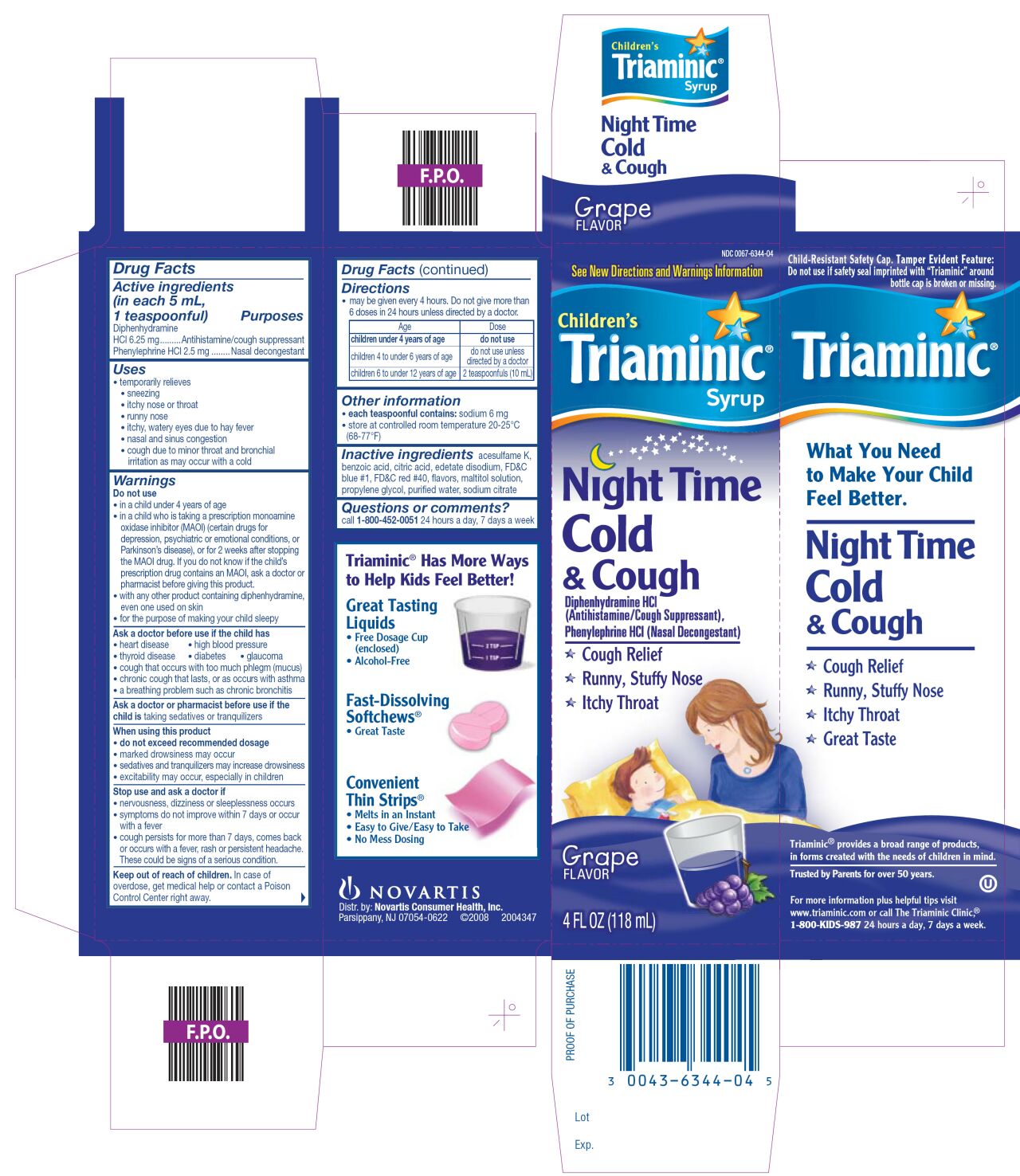
TRIAMINIC Childrens Night Time Cold and Cough
Dosage form: syrup
Ingredients: DIPHENHYDRAMINE HYDROCHLORIDE 6.25mg in 5mL, PHENYLEPHRINE HYDROCHLORIDE 2.5mg in 5mL
Labeler: Novartis Consumer Health, Inc.
NDC code: 0067-6344
Diphenhydramine HCl 6.25 mg
Phenylephrine HCl 2.5
Antihistamine/cough suppressant
Nasal decongestant
- temporarily relieves
• sneezing • itchy nose or throat • runny nose
• itchy, watery eyes due to hay fever • nasal and sinus congestion
• cough due to minor throat and bronchial irritation as may occur with a cold
- in a child under 4 years of age
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if the child’s prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- with any other product containing diphenhydramine, even one used on skin
- for the purpose of making your child sleepy
- heart disease • high blood pressure • thyroid disease
- diabetes • glaucoma
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts, or as occurs with asthma
- a breathing problem such as chronic bronchitis
taking sedatives or tranquilizers.
- do not exceed recommended dosage
- marked drowsiness may occur
- sedatives and tranquilizers may increase drowsiness
- excitability may occur, especially in children
- nervousness, dizziness or sleeplessness occurs
- symptoms do not improve within 7 days or occur with a fever
- cough persists for more than 7 days, comes back or occurs with a fever, rash or persistent headache. These could be signs of a serious condition.
In case of overdose, get medical help or contact a Poison Control Center right away.
- may be given every 4 hours. Do not give more than 6 doses in 24 hours unless directed by a doctor.
- find the right dose on chart below.
- use enclosed dosing cup only. Keep for use with this product only. Do not use any other dosing device
| Age | Dose |
| children under 4 years of age | do not use |
| children 4 to under 6 years of age | do not use unless directed by a doctor |
| children 6 to under 12 years of age | 2 teaspoonfuls (10 mL) |
- each teaspoonful contains: sodium 6 mg
- store at controlled room temperature 20-25°C (68-77°F)
acesulfame K, benzoic acid, citric acid, edetate disodium, FD&C blue #1, FD&C red #40, flavors, maltitol solution, propylene glycol, purified water, sodium citrate
call 1-800-452-0051 24 hours a day, 7 days a week
Child-Resistant Safety Cap
TAMPER-EVIDENT FEATURE:
DO NOT USE IF SAFETY SEAL IMPRINTED WITH “TRIAMINIC” AROUND BOTTLE CAP IS BROKEN OR MISSING.
For more information plus helpful tips visit www.triaminic.com
Distr. by: Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622 ©20xx
| TRIAMINIC
CHILDRENS NIGHT TIME COLD AND COUGH
diphenhydramine hcl, phenylephrine hcl syrup |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Novartis Consumer Health, Inc. (879821635) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Novartis Consumer Health | 129836151 | MANUFACTURE, ANALYSIS | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.