The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
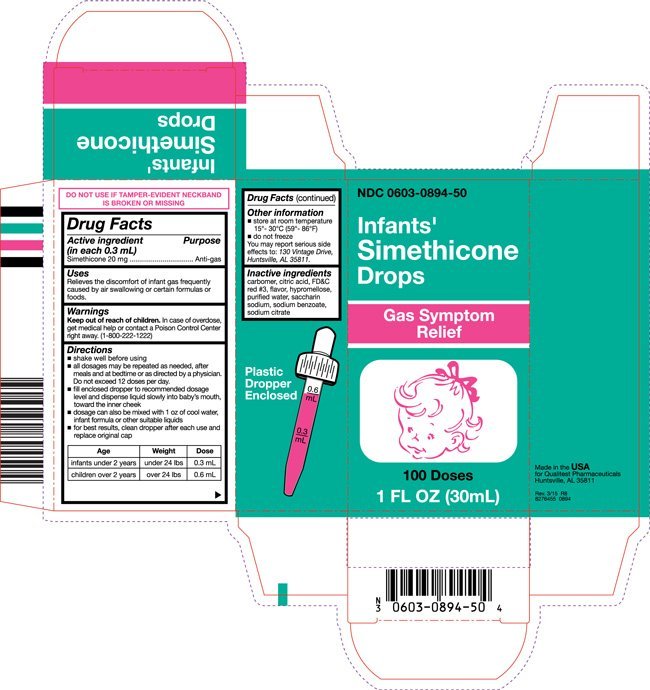
Infants Simethicone
Dosage form: solution/ drops
Ingredients: SIMETHICONE 63.3mg in 1mL, SILICON DIOXIDE 3.7mg in 1mL
Labeler: Qualitest Pharmaceuticals
NDC code: 0603-0894
Simethicone 20 mg
Anti-gas
Relieves the discomfort of infant gas frequently caused by air swallowing or certain formulas or foods.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
- shake well before using
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day.
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby’s mouth, toward the inner cheek
- dosage can also be mixed with 1 oz of cool water, infant formula or other suitable liquids
- for best results, clean dropper after each use and replace original cap
| Age | Weight | Dose |
| infants under 2 years | under 24 lbs | 0.3 mL |
| children over 2 years | over 24 lbs | 0.6 mL |
- store at room temperature 15°- 30°C (59°- 86°F)
- do not freeze
You may report serious side effects to: 130 Vintage Drive, Huntsville, AL 35811.
carbomer, citric acid, FD&C red #3, flavor, hypromellose, purified water, saccharin sodium, sodium benzoate, sodium citrate
Made in the USA
for Qualitest Pharmaceuticals
Huntsville, AL 35811
Rev. 3/15 R8
8276455 0894
| INFANTS SIMETHICONE
simethicone solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Qualitest Pharmaceuticals (011103059) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Vintage Pharmaceuticals-Huntsville | 825839835 | ANALYSIS(0603-0894), LABEL(0603-0894), MANUFACTURE(0603-0894), PACK(0603-0894) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.