Axe Excite Antiperspirant and Deodorant
Dosage form: stick
Ingredients: Aluminum Zirconium Tetrachlorohydrex GLY 17.8g in 100g
Labeler: Conopco Inc. d/b/a Unilever
NDC code: 64942-1131
Medically reviewed by Drugs.com. Last updated on Nov 1, 2024.
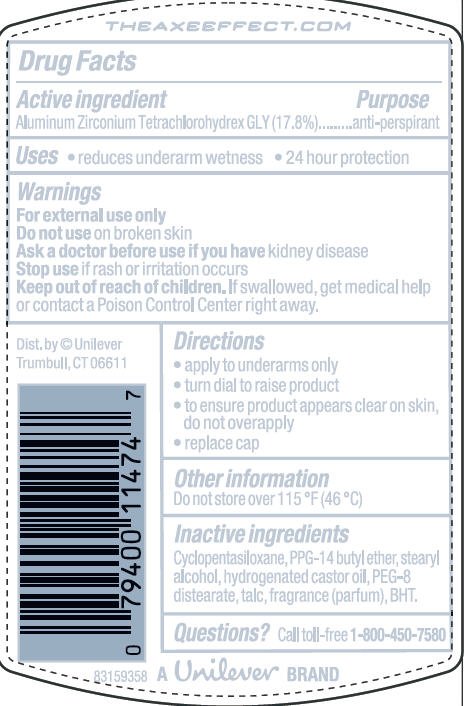
Active Ingredient
Aluminum Zirconium Tetrachlorohydrex GLY (17.8%)
Purpose
anti-perspirant
anti-perspirant
Uses · reduces underarm wetness ·24 hour protection
Warnings
For External Use Only
For External Use Only
Do not use on broken skin
Ask a doctor before use if you have kidney disease
Stop use if rash or irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
· apply to underarms only
· turn dial to raise product
· apply to underarms only
· turn dial to raise product
Inactive ingredients
Cyclopentasiloxane
PPG-14 Butyl Ether
Stearyl Alcohol
Hydrogenated Castor Oil
PEG-8 Distearate
Talc
Fragrance (Parfum)
BHT
Cyclopentasiloxane
PPG-14 Butyl Ether
Stearyl Alcohol
Hydrogenated Castor Oil
PEG-8 Distearate
Talc
Fragrance (Parfum)
BHT
Questions? Call toll-free 1-800-450-7580
2.7 oz front PDP


2.7 oz back PDP

| AXE
EXCITE ANTIPERSPIRANT AND DEODORANT
aluminum zirconium tetrachlorohydrex gly stick |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Conopco Inc. d/b/a Unilever (001375088) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Unilever Raeford HPCNA | 131411576 | manufacture | |
Document Id: bebe1da6-e40a-4d9b-9f0d-ab79ee7e406a
Set id: 4c3a6a60-4b1b-4a74-ac2d-ee015ef49dad
Version: 1
Conopco Inc. d/b/a Unilever
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
