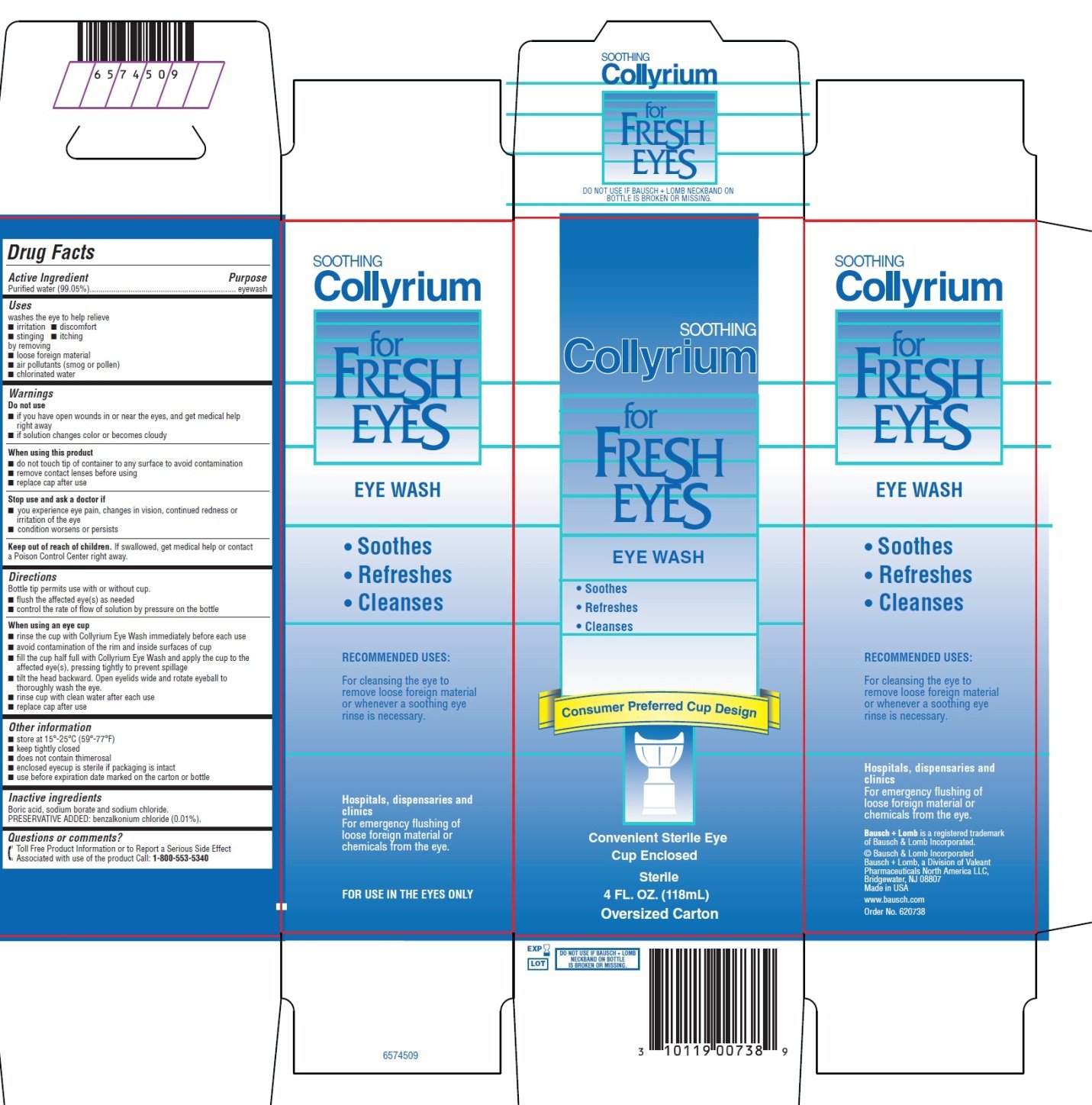
Collyrium for Fresh Eyes - Eye Wash
Dosage form: solution
Ingredients: WATER 99.05mL in 100mL
Labeler: Bausch & Lomb Incorporated
NDC code: 24208-073
Medically reviewed by Drugs.com. Last updated on Nov 25, 2024.
Purified water (99.05%)
eyewash
washes the eye to help relieve
- •
- irritation
- •
- discomfort
- •
- stinging
- •
- itching
by removing
- •
- loose foreign material
- •
- air pollutants (smog or pollen)
- •
- chlorinated water
- •
- if you have open wounds in or near the eyes, and get medical help right away
- •
- if solution changes color or becomes cloudy
- •
- do not touch tip of container to any surface to avoid contamination
- •
- remove contact lenses before using
- •
- replace cap after use
- •
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- •
- condition worsens or persists
If swallowed, get medical help or contact a Poison Control Center right away.
Bottle tip permits use with or without cup.
- •
- flush the affected eye(s) as needed
- •
- control the rate of flow of solution by pressure on the bottle
- •
- rinse the cup with Collyrium Eye Wash immediately before each use
- •
- avoid contamination of the rim and inside surfaces of cup
- •
- fill the cup half full with Collyrium Eye Wash and apply the cup to the affected eye(s), pressing tightly to prevent spillage
- •
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye.
- •
- rinse cup with clean water after each use
- •
- replace cap after use
- •
- store at 15°-25°C (59°-77°F)
- •
- keep tightly closed
- •
- does not contain thimerosal
- •
- enclosed eyecup is sterile if packaging is intact
- •
- use before expiration date marked on the carton or bottle
Boric acid, sodium borate and sodium chloride.
PRESERVATIVE ADDED: benzalkonium chloride (0.01%).
Toll Free Product Information or to Report a Serious Side Effect Associated with use of the product
Call: 1-800-553-5340
6574509
Soothing Collyrium
for
FRESH
EYES
EYE WASH
- •
- Soothes
- •
- Refreshes
- •
- Cleanses
Consumer Preferred Cup Design
Convenient Sterile Eye
Cup Enclosed
Sterile
4 FL. OZ. (118mL)
Oversized Carton
| COLLYRIUM FOR FRESH EYES - EYE WASH
water solution |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - Bausch & Lomb Incorporated (196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bausch & Lomb Incorporated | 114406598 | MANUFACTURE(24208-073) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bausch & Lomb Incorporated | 079587625 | MANUFACTURE(24208-073) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
