The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
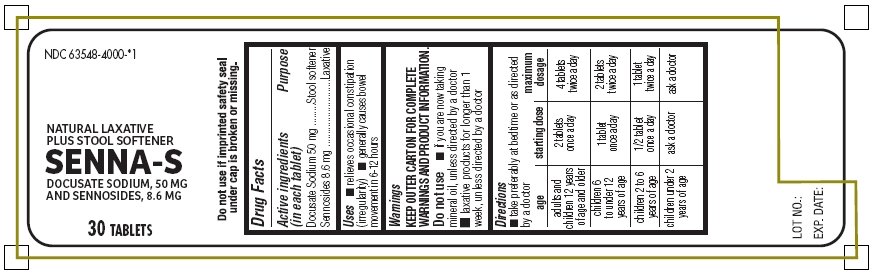
Senna-S
Dosage form: tablet, coated
Ingredients: DOCUSATE SODIUM 50mg, SENNOSIDES 8.6mg
Labeler: Avema Pharma Solutions
NDC code: 63548-4001
Docusate Sodium 50 mg*
Sennosides 8.6 mg**
*Stool softener
**Laxative
- relieves occasional constipation (irregularity)
- generally causes bowel movement in 6 - 12 hours
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week, unless directed by a doctor
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may be signs of a serious condition.
If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years of age and older | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years of age | 1 tablet once a day | 2 tablets twice a day |
| children 2 to 6 years of age | ½ tablet once a day | 1 tablet twice a day |
| children under 2 years of age | ask a doctor | ask a doctor |
- each tablet contains: sodium 6 mg/tablet VERY LOW SODIUM
- each tablet contains: calcium 20 mg/tablet
- store at 15°-30°C (59°-86°F) in well closed containers
- do not use if imprinted safety seal under cap is broken or missing
- *This product is not manufactured or distributed by Purdue Pharma L.P., owner of the registered trademark Senokot-S®.
carnauba wax, colloidal silicon dioxide,croscarmellose sodium, dibasic calcium phosphate dihydrate, D&C Yellow #10 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol (PEG) 8000, sodium benzoate, stearic acid, and titanium dioxide. May also contain: maltodextrin, mineral oil, Opadry II Orange, pregelatinized starch, talc, silicon dioxide 50s.
| SENNA-S
docusate sodium and sennosides tablet, coated |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Avema Pharma Solutions (804087749) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.