GSI 1 Pill: green, capsule/oblong, 20mm
Generic Name: cobicistat/elvitegravir/emtricitabine/tenofovir disoproxil
The pill with imprint GSI 1 (Green, Capsule/Oblong, 20mm) has been identified as Stribild cobicistat 150 mg/elvitegravir 150 mg/emtricitabine 200 mg/tenofovir disoproxil fumarate 300 mg and is used for HIV Infection. It belongs to the drug class antiviral combinations and is not a controlled substance.
Images for GSI 1

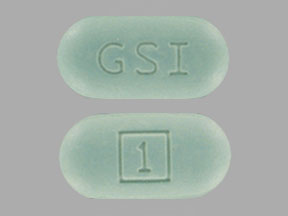

Stribild
- Generic Name
- cobicistat/elvitegravir/emtricitabine/tenofovir disoproxil
- Imprint
- GSI 1
- Strength
- cobicistat 150 mg/elvitegravir 150 mg/emtricitabine 200 mg/tenofovir disoproxil fumarate 300 mg
- Color
- Green
- Size
- 20.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Antiviral combinations
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Gilead Sciences, Inc.
- Inactive Ingredients
-
microcrystalline cellulose,
croscarmellose sodium,
magnesium stearate,
silicon dioxide,
sodium lauryl sulfate,
lactose monohydrate,
titanium dioxide,
magnesium silicate,
FD&C Blue No. 2,
aluminum oxide,
ferric oxide yellow
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 61958-1201 | Gilead Sciences, Inc. |
| 54569-6352 | A-S Medication Solutions, LLC (repackager) |
| 54868-6371 | Physicians Total Care Inc. (repackager) |
Related images for "GSI 1"
More about Stribild (cobicistat / elvitegravir / emtricitabine / tenofovir disoproxil)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (88)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- FDA approval history
- Drug class: antiviral combinations
- En español
Patient resources
Professional resources
- Stribild prescribing information
- Elvitegravir, Cobicistat, Emtricitabine, and tenofovir Disoproxil Fumarate (AHFS Monograph)
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.