TNKase Dosage
Generic name: TENECTEPLASE 50mg in 10mL;
Dosage form: injection
Drug class: Thrombolytics
Medically reviewed by Drugs.com. Last updated on Apr 4, 2025.
Recommended Dosage
Initiate treatment as soon as possible after the onset of STEMI symptoms.
TNKase is for intravenous (IV) administration only, administered as a single bolus over 5 seconds. Individualize dosage based on the patient's weight (see Table 1).
| Patient Weight (kg) |
TNKase (mg) |
Volume TNKase* to be administered (mL) |
|---|---|---|
|
||
| < 60 | 30 | 6 |
| ≥ 60 to < 70 | 35 | 7 |
| ≥ 70 to < 80 | 40 | 8 |
| ≥ 80 to < 90 | 45 | 9 |
| ≥ 90 | 50 | 10 |
Preparation
Follow the below steps to prepare TNKase for administration:
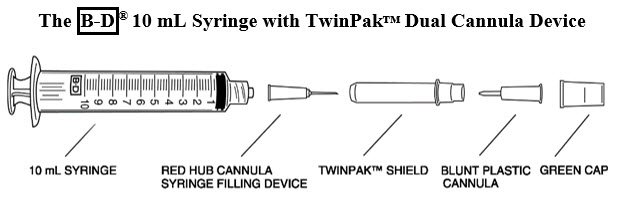
Remove the shield assembly from the supplied B-D® 10 mL syringe with TwinPak™ Dual Cannula Device (see Figure 1) and aseptically withdraw 10 mL of Sterile Water for Injection, USP, from the supplied diluent vial using the red hub cannula syringe filling device. Only use the supplied Sterile Water for Injection, USP for reconstitution.
- Note: Do not discard the shield assembly.
- Aseptically reconstitute the vial with 10 mL Sterile Water for Injection, USP by directing the stream into the lyophilized powder to obtain a final concentration of 5 mg/mL. Slight foaming upon reconstitution is not unusual; any large bubbles will dissipate if the product is allowed to stand undisturbed for several minutes.
- Gently swirl until contents are completely dissolved. DO NOT SHAKE. The reconstituted preparation results in a colorless to pale yellow transparent solution.
- Determine the appropriate dose of TNKase and withdraw this volume (in milliliters) from the reconstituted vial with the syringe. Discard any unused solution.
- Stand the shield vertically on a flat surface (with green side down) and passively recap the red hub cannula.
- Remove the entire shield assembly, including the red hub cannula, by twisting counterclockwise. Note: The shield assembly also contains the clear-ended blunt plastic cannula; retain for split septum intravenous access.
Administration
Follow the below steps for administration of TNKase;
- Inspect the product prior to administration for particulate matter and discoloration. Administer TNKase as reconstituted at 5 mg/mL.
- Precipitation may occur when TNKase is administered in an intravenous line containing dextrose. Flush dextrose-containing lines with a saline-containing solution prior to and following single bolus administration of TNKase.
- Administer reconstituted TNKase as a single intravenous bolus over 5 seconds.
- Because TNKase contains no antibacterial preservatives, reconstitute immediately before use. If the reconstituted TNKase is not used immediately, refrigerate the TNKase vial at 2°C to 8°C (36°F to 46°F) and use within 8 hours.
- Although the supplied syringe is compatible with a conventional needle, this syringe is designed to be used with needleless intravenous systems. From the information below, follow the instructions applicable to the intravenous system in use.
Split septum intravenous system: - Remove the green cap.
- Attach the clear-ended blunt plastic cannula to the syringe.
- Remove the shield and use the blunt plastic cannula to access the split septum injection port.
- Because the blunt plastic cannula has two side ports, air or fluid expelled through the cannula will exit in two sideways directions; direct away from face or mucous membranes.
Luer-Lok® system: Connect syringe directly to intravenous port. Conventional needle
(not supplied in this kit):Attach a large bore needle, e.g., 18 gauge, to the syringe's universal Luer-Lok®. - Dispose of the syringe, cannula and shield per established procedures.
Frequently asked questions
More about TNKase (tenecteplase)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- During pregnancy
- FDA approval history
- Drug class: thrombolytics
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

