Koate Dosage
Generic name: Antihemophilic Factor Human 250[iU] in 5mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Dec 13, 2023.
For intravenous use after reconstitution only.
Dose
- Dose and duration of treatment depend on the severity of the Factor VIII deficiency, location and extent of bleeding, and the patient’s clinical condition.
- Each vial of KOĀTE is labeled with the actual Factor VIII potency in international units (IU). Calculation of the required dose of Factor VIII is based on the empirical finding that one IU of Factor VIII per kg body weight raises the plasma Factor VIII activity by approximately 2% of normal activity or 2 IU/dL.
- The required dose can be determined using the following formula:
Dose (IU) = Body Weight (kg) x Desired Factor VIII Rise (% normal or IU/dL) x 0.5
- Estimate the expected in vivo peak increase in Factor VIII level, expressed as IU/dL (or % normal), using the following formula:
Estimated Increment of Factor VIII
(% normal or IU/dL) = [Total Dose (IU)/Body Weight (kg)] x 2
- Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses. Base the dose and frequency on the individual clinical response.
Control and Prevention of Bleeding Episodes
A guide for dosing KOĀTE for the control and prevention of bleeding episodes (1,2) is provided in Table 1. Consideration should be given to maintaining a Factor VIII activity at or above the target range.
| Type of Bleeding | Factor VIII:C Level Required (% of normal) |
Doses (IU/kg) |
Frequency of Doses (hours) |
Duration of Therapy (days) |
| Minor Large bruises Significant cuts or scrapes Uncomplicated joint hemorrhage |
30 | 15 | 12 (twice daily) |
Until hemorrhage stops and healing has been achieved (1–2 days). |
| Moderate Nose, mouth and gum bleeds Dental extractions Hematuria |
50 | 25 | 12 (twice daily) |
Until healing has been achieved (2–7 days, on average). |
| Major Joint hemorrhage Muscle hemorrhage Major trauma Hematuria Intracranial and intraperitoneal bleeding |
80-100 | Initial: 40-50 Maintenance: 25 |
12 (twice daily) |
For at least 3–5 days Until healing has been achieved for up to 10 days. Intracranial hemorrhage may require prophylaxis therapy for up to 6 months. |
| Surgery | Prior to surgery: 80-100 After surgery: 60-100 |
40-50 30-50 |
Once 12 (twice daily) |
Prior to surgery For the next 7–10 days, or until healing has been achieved. |
Preparation and Reconstitution
- Use aseptic technique (clean and sanitized) and a flat work surface during the reconstitution procedure.
- Bring the vials of KOĀTE and the diluent (Sterile Water for Injection) to room temperature before use.
- Remove the shrink band from the KOĀTE vial. Do not use KOĀTE if the shrink band is absent or shows signs of tampering, and notify Grifols Therapeutics LLC immediately.
- Remove the plastic cap from the KOĀTE vial and clean the top of the stopper with an alcohol swab. Allow the stopper to dry.
- Repeat this step with the vial of sterile water.
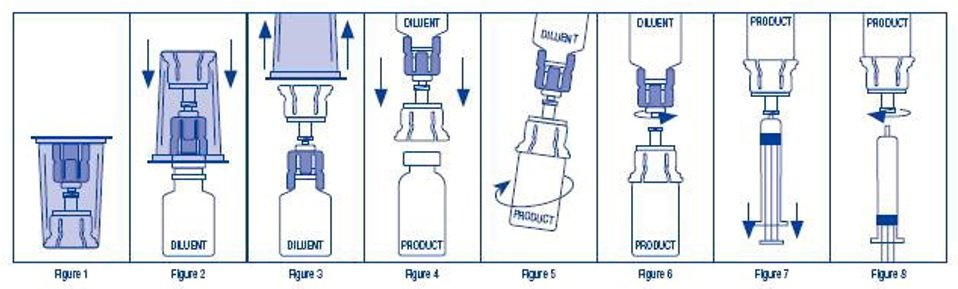
- Open the sterile Mix2Vial® package by peeling away the lid (Figure 1). Do not remove the device from the package.
- Place the diluent vial upright on an even surface. Holding the diluent vial securely, push the blue end of the Mix2Vial straight down until the spike penetrates the stopper (Figure 2).
- Remove the clear outer packaging from the Mix2Vial and discard it (Figure 3).
- Place the KOĀTE vial upright on a flat surface, and invert the diluent vial with the Mix2Vial still attached.
- While holding the KOĀTE vial securely on a flat surface, push the clear end of the Mix2Vial straight down until the spike penetrates the stopper (Figure 4). The diluent will automatically transfer into the KOĀTE vial by the vacuum contained within it.
Note: If the Mix2Vial is connected at an angle, the vacuum may be released from the product vial and the diluent will not transfer into the product vial. If vacuum is lost, use a sterile syringe and needle to remove the sterile water from the diluent vial and inject it into the KOĀTE vial, directing the stream of fluid against the wall of the vial. - With the diluent and KOĀTE vials still attached to the Mix2Vial, agitate vigorously for 10 to 15 seconds, then gently swirl (Figure 5) until the powder is completely dissolved. Avoid excessive foaming. The reconstituted solution should be clear to opalescent. Do not use if particulate matter or discoloration is observed.
- Remove the diluent vial and the blue end of the Mix2Vial (Figure 6) by holding each side of the vial adapter and twisting counterclockwise.
- Draw air into an empty, sterile syringe. Connect the syringe to the clear end of the Mix2Vial by pressing and twisting clockwise, and push the air into the KOĀTE vial.
- Immediately invert the system upside down and then draw the reconstituted KOĀTE into the syringe by pulling the plunger back slowly (Figure 7).
- Detach the filled syringe from the Mix2Vial by turning counter-clockwise (Figure 8). Use KOĀTE within 3 hours after reconstitution. Do not refrigerate after reconstitution.
Administration
For intravenous administration only
- If the dose requires more than one vial of KOĀTE:
- Reconstitute each vial using a new Mix2Vial.
- Draw up all the solution into a single syringe.
- Visually inspect the final solution for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed.
- Attach the syringe to the connector end of an infusion set.
- Administer intravenously. The rate of administration should be determined by the patient’s comfort level, and no faster than 10 mL per minute.
More about Koate (antihemophilic factor)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
Patient resources
Other brands
Advate, Altuviiio, Eloctate, Esperoct, ... +15 more
Professional resources
Other brands
Advate, Altuviiio, Eloctate, Esperoct, ... +12 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.