Advate Dosage
Generic name: ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 125[iU] in 1mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Apr 3, 2025.
For intravenous injection after reconstitution only.
Dose
- Dosage and duration of treatment depend on the severity of factor VIII deficiency, the location and extent of the bleeding, and the patient's clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening bleeding episodes.
- Each vial of ADVATE has the recombinant factor VIII potency in International Units (IU) stated on the label. The expected in vivo peak increase in factor VIII level expressed as IU/dL of plasma or percent of normal can be estimated using the following formulas:
IU/dL (or % of normal) = [total dose (IU)/body weight (kg)] × 2 [IU/dL]/[IU/kg]
OR
Required dose (International Units) = body weight (kg) × desired factor VIII rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
Examples (assuming patient's baseline factor VIII level is <1% of normal):
- A dose of 1750 IU ADVATE administered to a 70 kg patient should be expected to result in a peak postinfusion factor VIII increase of: 1750 IU × {[2 IU/dL]/[IU/kg]}/[70 kg] = 50 IU/dL (50% of normal).
- A peak level of 70% is required in a 40 kg child. In this situation, the appropriate dose would be 40 kg × 70 IU/dL/{[2 IU/dL]/[IU/kg]} = 1400 IU.
- Base the dose and frequency on the individual clinical response. Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses to ADVATE. Although the dose can be estimated by the calculations above, whenever possible, perform appropriate laboratory tests including serial factor VIII activity assays.
Control and Prevention of Bleeding Episodes
A guide for dosing ADVATE for the control and prevention of bleeding episodes is provided in Table 1. The goal of treatment is to maintain a plasma factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in Table 1.
| Type of Bleeding Episodes | Factor VIII Level Required (% of normal or IU/dL) |
Dose* (IU/kg) |
Frequency of Doses (hours) |
Duration of Therapy (days) |
|---|---|---|---|---|
|
||||
| Minor Early hemarthrosis, mild muscle bleeding, or mild oral bleeding episode. |
20 to 40 | 10 to 20 | 12 to 24 (Every 8 to 24 hours for patients under the age of 6) |
Until the bleeding is resolved (approximately 1 to 3 days). |
| Moderate Muscle bleeding, bleeding into the oral cavity, definite hemarthroses, and known trauma. |
30 to 60 | 15 to 30 | 12 to 24 (Every 8 to 24 hours for patients under the age of 6) |
Until the bleeding is resolved (approximately 3 days or more). |
| Major Significant gastrointestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, central nervous system bleeding, bleeding in the retropharyngeal or retroperitoneal spaces or iliopsoas sheath, fractures, head trauma. |
60 to 100 | 30 to 50 | 8 to 24 (Every 6 to 12 hours for patients under the age of 6) |
Until bleeding is resolved. |
Perioperative Management
A guide for dosing ADVATE during surgery (perioperative management) is provided in Table 2. The goal of treatment is to maintain a plasma factor VIII activity level at or above the plasma level (in % of normal or in IU/dL) outlined in Table 2.
| Type of Surgery | Factor VIII Level Required (% of normal or IU/dL) |
Dose* (IU/kg) |
Frequency of Doses (hours) |
Duration of Therapy (days) |
|---|---|---|---|---|
|
||||
| Minor Including tooth extraction |
60 to 100 | 30 to 50 | Single dose within 1 hour of the operation. | Single dose or repeat until bleeding is resolved. |
| 12 to 24 (as needed to control bleeding) | For dental procedures, adjunctive therapy may be considered. | |||
| Major Intracranial, intra-abdominal, or intrathoracic surgery, joint replacement surgery |
80 to 120 (pre- and postoperative) |
40 to 60 | One dose preoperative to achieve 100% activity. Every 8 to 24 to keep factor VIII activity in desired range. (Every 6 to 24 hours for patients under the age of 6) |
Until healing is complete. |
Preparation and Reconstitution
Preparation
- Do not remove ADVATE or diluent vials from the external housing.
- Always work on a clean surface and wash your hands before performing the procedures.
- Examine the packaging containing ADVATE to ensure no damage or peeling of the lid is evident. Do not use if the lid is not completely sealed on the blister. Do not remove ADVATE or diluent vials from the external housing.
Reconstitution
- Allow the ADVATE package to reach room temperature.
- Open the package by peeling away the lid. Remove ADVATE from the package and verify that the expiration date on the label has not passed and the potency unit number is same as expected. Inspect parenteral drug products for discoloration and particulate matter. The ADVATE powder should be white to off-white in color and the diluent free from foreign particles. Do not use if the criteria are not met.
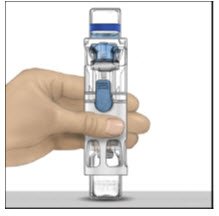
- Place the ADVATE on a flat surface with the diluent vial on top (Figure A). The diluent vial has a blue stripe. Do not remove the blue cap until instructed in a later step.
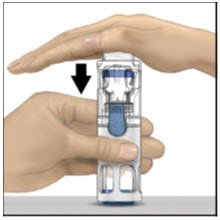
- With one hand holding the ADVATE housing, press down firmly on the diluent vial with the other hand until the system is fully collapsed and the diluent flows down into the ADVATE vial (Figure B). Do not tilt the system until the transfer is complete.
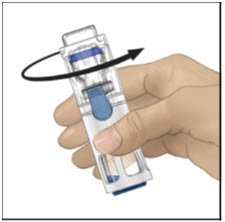
- Verify that diluent transfer is complete. Swirl gently until the powder is completely dissolved (Figure C). Do not shake. Do not refrigerate after reconstitution.
| Figure A | Figure B | Figure C |
 |
 |
 |
Administration
For intravenous injection after reconstitution only.
- Inspect parenteral drug products for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance. If not, do not use the solution and notify Takeda Pharmaceuticals U.S.A., Inc. immediately.
- Administer ADVATE at room temperature within 3 hours of reconstitution.
- Use plastic syringes with this product because proteins in the product tend to stick to the surface of glass syringes.
- Use aseptic technique.
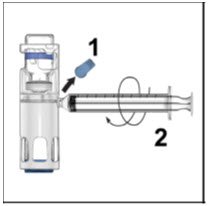
- Remove the blue cap from the housing. Connect the syringe to the system (Figure D). Do not inject air into the ADVATE.
- Turn the system upside down (factor concentrate vial now on top). Draw the factor concentrate into the syringe by pulling the plunger back slowly (Figure E).
- Disconnect the syringe, attach a suitable needle, and inject intravenously as instructed. If a patient is to receive more than one ADVATE-BAXJECT® III system or a combination of an ADVATE-BAXJECT II and an ADVATE-BAXJECT III system, the contents may be drawn into the same syringe.
- Administer ADVATE over a period of ≤5 minutes (maximum infusion rate 10 mL/min). Determine the pulse rate before and during administration of ADVATE. Should a significant increase in pulse rate occur, reducing the rate of administration or temporarily halting the injection usually allows the symptoms to disappear promptly.
| Figure D | Figure E |
 |
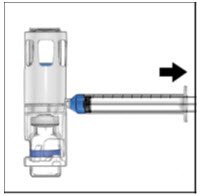 |
More about Advate (antihemophilic factor)
- Check interactions
- Compare alternatives
- Reviews (1)
- Side effects
- During pregnancy
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Other brands
Altuviiio, Eloctate, Esperoct, Nuwiq, ... +14 more
Professional resources
Other brands
Altuviiio, Eloctate, Esperoct, Nuwiq, ... +12 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

