Depo-Provera Contraceptive: Package Insert / Prescribing Info
Package insert / product label
Generic name: medroxyprogesterone acetate
Dosage form: injection, suspension
Drug classes: Contraceptives, Hormones / antineoplastics, Progestogens
J Code (medical billing code): J1050 (1 mg, injection)
Medically reviewed by Drugs.com. Last updated on Aug 5, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
DEPO-PROVERA CI (medroxyprogesterone acetate) injectable suspension, for intramuscular use
Initial U.S. Approval: 1959
WARNING: LOSS OF BONE MINERAL DENSITY
See full prescribing information for complete boxed warning.
- •
- Women who use Depo-Provera Contraceptive Injection (Depo-Provera CI) may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible. (5.1)
- •
- It is unknown if use of Depo-Provera CI during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life. (5.1)
- •
- Depo-Provera CI is not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate. (1, 5.1)
Recent Major Changes
|
Contraindications, Pregnancy (4) |
Removed 04/2024 |
Indications and Usage for Depo-Provera Contraceptive
- •
- Depo-Provera CI is a progestin indicated for use by females of reproductive potential to prevent pregnancy. (1)
Limitations of Use:
The use of Depo-Provera CI is not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate. (1, 5.1)
Depo-Provera Contraceptive Dosage and Administration
- •
- The recommended dose is 150 mg of Depo-Provera CI every 3 months (13 weeks) administered by deep, intramuscular (IM) injection in the gluteal or deltoid muscle. (2.1)
Dosage Forms and Strengths
Contraindications
- •
- Active thrombophlebitis, or current or past history of thromboembolic disorders, or cerebral vascular disease. (4)
- •
- Known or suspected malignancy of breast. (4)
- •
- Known hypersensitivity to Depo-Provera CI (medroxyprogesterone acetate or any of its other ingredients). (4)
- •
- Significant liver disease. (4)
- •
- Undiagnosed vaginal bleeding. (4)
Warnings and Precautions
- •
- Thromboembolic Disorders: Discontinue Depo-Provera CI in patients who develop thrombosis. (5.2)
- •
- Cancer Risks: Monitor women with a strong family history of breast cancer carefully. (5.3)
- •
- Ectopic Pregnancy: Consider ectopic pregnancy if a woman using Depo-Provera CI becomes pregnant or complains of severe abdominal pain. (5.4)
- •
- Anaphylaxis and Anaphylactoid Reactions: Provide emergency medical treatment. (5.5)
- •
- Liver Function: Discontinue Depo-Provera CI if jaundice or disturbances of liver function develop. (5.7)
- •
- Carbohydrate Metabolism: Monitor diabetic patients carefully. (5.12)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence >5%): menstrual irregularities (bleeding or spotting) 57% at 12 months, 32% at 24 months, abdominal pain/discomfort 11%, weight gain >10 lb at 24 months 38%, dizziness 6%, headache 17%, nervousness 11%, decreased libido 6%. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Drugs or herbal products that induce certain enzymes, including CYP3A4, may decrease the effectiveness of contraceptive drug products. Counsel patients to use a back-up method or alternative method of contraception when enzyme inducers are used with Depo-Provera CI. (7.1)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
Full Prescribing Information
WARNING: LOSS OF BONE MINERAL DENSITY
- •
- Women who use Depo-Provera Contraceptive Injection (Depo-Provera CI) may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible [see Warnings and Precautions (5.1)].
- •
- It is unknown if use of Depo-Provera CI during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life [see Warnings and Precautions (5.1)].
- •
- Depo-Provera CI is not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate [see Indications and Usage (1) and Warnings and Precautions (5.1)].
1. Indications and Usage for Depo-Provera Contraceptive
Depo-Provera CI is indicated for use by females of reproductive potential to prevent pregnancy.
Limitations of Use:
The use of Depo-Provera CI is not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
2. Depo-Provera Contraceptive Dosage and Administration
2.1 Prevention of Pregnancy
Both the 1 mL vial and the 1 mL prefilled syringe of Depo-Provera CI should be vigorously shaken just before use to ensure that the dose being administered represents a uniform suspension.
The recommended dose is 150 mg of Depo-Provera CI every 3 months (13 weeks) administered by deep intramuscular (IM) injection using strict aseptic technique in the gluteal or deltoid muscle, rotating the sites with every injection. As with any IM injection, to avoid an inadvertent subcutaneous injection, body habitus should be assessed prior to each injection to determine if a longer needle is necessary particularly for gluteal IM injection.
Use for longer than 2 years is not recommended (unless other birth control methods are considered inadequate) due to the impact of long-term Depo-Provera CI treatment on bone mineral density (BMD) [see Warnings and Precautions (5.1)]. Dosage does not need to be adjusted for body weight [see Clinical Studies (14.1)].
To ensure the patient is not pregnant at the time of the first injection, the first injection should be given ONLY during the first 5 days of a normal menstrual period or within the first 5-days post‑partum. In post‑partum mothers who exclusively breastfeed, administer Depo‑Provera CI during or after the sixth post‑partum week. If the time interval between injections is greater than 13 weeks, the physician should determine that the patient is not pregnant before administering the drug. The efficacy of Depo-Provera CI depends on adherence to the dosage schedule of administration.
2.2 Switching from Other Methods of Contraception
When switching from other contraceptive methods, Depo-Provera CI should be given in a manner that ensures continuous contraceptive coverage based upon the mechanism of action of both methods, (e.g., patients switching from oral contraceptives should have their first injection of Depo-Provera CI on the day after the last active tablet or at the latest, on the day following the final inactive tablet).
3. Dosage Forms and Strengths
Sterile Aqueous suspension: 150 mg/mL
Prefilled syringes are available packaged with 22-gauge x 1 1/2 inch Terumo® SurGuard™ Needles.
4. Contraindications
The use of Depo-Provera CI is contraindicated in the following conditions:
- •
- Active thrombophlebitis, or current or history of thromboembolic disorders, or cerebral vascular disease [see Warnings and Precautions (5.2)].
- •
- Known or suspected malignancy of breast [see Warnings and Precautions (5.3)].
- •
- Known hypersensitivity to Depo-Provera CI (medroxyprogesterone acetate) or any of its other ingredients [see Warnings and Precautions (5.5)].
- •
- Significant liver disease [see Warnings and Precautions (5.7)].
- •
- Undiagnosed vaginal bleeding [see Warnings and Precautions (5.10)].
5. Warnings and Precautions
5.1 Loss of Bone Mineral Density
Use of Depo-Provera CI reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of Depo-Provera CI by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
A study to assess the reversibility of loss of BMD in adolescents was conducted with Depo-Provera CI. After discontinuing Depo-Provera CI in these adolescents, mean BMD loss at the total hip and femoral neck did not fully recover by 5 years (60 months) post-treatment in the sub-group of adolescents who were treated for more than 2 years [see Clinical Studies (14.3)]. Similarly, in adults, there was only partial recovery of mean BMD at the total hip, femoral neck, and lumbar spine towards baseline by 2 years post-treatment [see Clinical Studies (14.2)].
The use of Depo-Provera CI is not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate. BMD should be evaluated when a woman needs to continue to use Depo-Provera CI long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods should be considered in the risk/benefit analysis for the use of Depo-Provera CI in women with osteoporosis risk factors. Depo-Provera CI can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids).
5.2 Thromboembolic Disorders
There have been reports of serious thrombotic events in women using Depo-Provera CI (150 mg). However, Depo-Provera CI has not been causally associated with the induction of thrombotic or thromboembolic disorders. Any patient who develops thrombosis while undergoing therapy with Depo-Provera CI should discontinue treatment unless she has no other acceptable options for birth control.
Do not re-administer Depo-Provera CI pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. Do not re-administer if examination reveals papilledema or retinal vascular lesions.
5.3 Cancer Risks
Breast Cancer
Women who have or have had a history of breast cancer should not use hormonal contraceptives, including Depo-Provera CI, because breast cancer may be hormonally sensitive [see Contraindications (4)]. Women with a strong family history of breast cancer should be monitored with particular care.
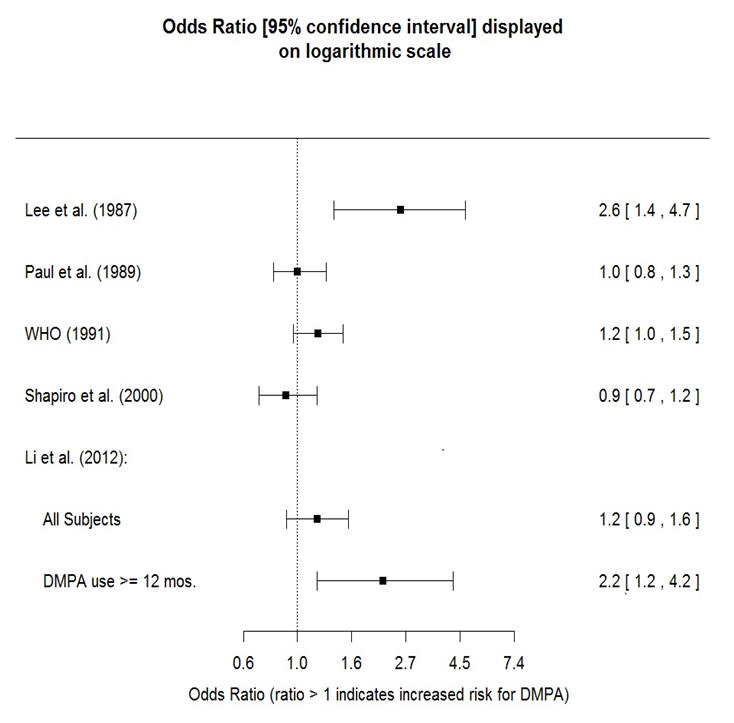
The results of five large case-control studies assessing the association between depo-medroxyprogesterone acetate (DMPA) use and the risk of breast cancer are summarized in Figure 1. Three of the studies suggest a slightly increased risk of breast cancer in the overall population of users; these increased risks were statistically significant in one study. One recent US study1 evaluated the recency and duration of use and found a statistically significantly increased risk of breast cancer in recent users (defined as last use within the past five years) who used DMPA for 12 months or longer; this is consistent with results of a previous study2.
Figure 1. Risk estimates for breast cancer in DMPA users
Based on the published SEER-18 2011 incidence rate (age-adjusted to the 2000 US Standard Population) of breast cancer for US women, all races, age 20 to 49 years, a doubling of risk would increase the incidence of breast cancer in women who use Depo-Provera CI from about 72 to about 144 cases per 100,000 women.
Cervical Cancer
A statistically nonsignificant increase in relative risk (RR) estimates of invasive squamous-cell cervical cancer has been associated with the use of Depo-Provera CI in women who were first exposed before the age of 35 years (RR 1.22 to 1.28 and 95% CI 0.93 to 1.70). The overall, nonsignificant RR of invasive squamous-cell cervical cancer in women who ever used Depo-Provera CI was estimated to be 1.11 (95% CI 0.96 to 1.29). No trends in risk with duration of use or times since initial or most recent exposure were observed.
5.4 Ectopic Pregnancy
Be alert to the possibility of an ectopic pregnancy among women using Depo-Provera CI who become pregnant or complain of severe abdominal pain.
5.5 Anaphylaxis and Anaphylactoid Reaction
Anaphylaxis and anaphylactoid reaction have been reported with the use of Depo-Provera CI. Institute emergency medical treatment if an anaphylactic reaction occurs.
5.6 Injection Site Reactions
Injection site reactions have been reported with use of Depo-Provera CI [see Adverse Reactions (6.2)]. Persistent injection site reactions may occur after administration of Depo-Provera CI due to inadvertent subcutaneous administration or release of the drug into the subcutaneous space while removing the needle [see Dosage and Administration (2.1)].
5.7 Liver Function
Discontinue Depo-Provera CI use if jaundice or acute or chronic disturbances of liver function develop. Do not resume use until markers of liver function return to normal and Depo-Provera CI causation has been excluded.
5.8 Convulsions
There have been a few reported cases of convulsions in patients who were treated with Depo-Provera CI. Association with drug use or pre-existing conditions is not clear.
5.9 Depression
Monitor patients who have a history of depression and do not re-administer Depo-Provera CI if depression recurs.
5.10 Bleeding Irregularities
Most women using Depo-Provera CI experience disruption of menstrual bleeding patterns. Altered menstrual bleeding patterns include amenorrhea, irregular or unpredictable bleeding or spotting, prolonged spotting or bleeding, and heavy bleeding. Rule out the possibility of organic pathology if abnormal bleeding persists or is severe, and institute appropriate treatment.
As women continue using Depo-Provera CI, fewer experience irregular bleeding and more experience amenorrhea. In clinical studies of Depo-Provera CI, by month 12 amenorrhea was reported by 55% of women, and by month 24, amenorrhea was reported by 68% of women using Depo-Provera CI.
5.11 Weight Gain
Women tend to gain weight while on therapy with Depo-Provera CI. From an initial average body weight of 136 lb, women who completed 1 year of therapy with Depo-Provera CI gained an average of 5.4 lb. Women who completed 2 years of therapy gained an average of 8.1 lb. Women who completed 4 years gained an average of 13.8 lb. Women who completed 6 years gained an average of 16.5 lb. Two percent of women withdrew from a large-scale clinical trial because of excessive weight gain.
5.12 Carbohydrate Metabolism
A decrease in glucose tolerance has been observed in some patients on Depo-Provera CI treatment. Monitor diabetic patients carefully while receiving Depo-Provera CI.
5.13 Fluid Retention
Because progestational drugs including Depo-Provera CI may cause some degree of fluid retention, monitor patients with conditions that might be influenced by this condition, such as epilepsy, migraine, asthma, and cardiac or renal dysfunction.
5.14 Return of Fertility
Return to ovulation and fertility is likely to be delayed after stopping Depo-Provera CI. In a large US study of women who discontinued use of Depo-Provera CI to become pregnant, data are available for 61% of them. Of the 188 women who discontinued the study to become pregnant, 114 became pregnant. Based on Life-Table analysis of these data, it is expected that 68% of women who do become pregnant may conceive within 12 months, 83% may conceive within 15 months, and 93% may conceive within 18 months from the last injection. The median time to conception for those who do conceive is 10 months following the last injection with a range of 4 to 31 months, and is unrelated to the duration of use. No data are available for 39% of the patients who discontinued Depo-Provera CI to become pregnant and who were lost to follow-up or changed their mind.
5.15 Sexually Transmitted Infections
Patients should be counseled that Depo-Provera CI does not protect against HIV infection (AIDS) and other sexually transmitted infections.
5.16 Monitoring
A woman who is taking hormonal contraceptive should have a yearly visit with her healthcare professional for a blood pressure check and for other indicated healthcare.
5.17 Interference with Laboratory Tests
The use of Depo-Provera CI may change the results of some laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins [see Drug Interactions (7.2)].
6. Adverse Reactions/Side Effects
The following important adverse reactions observed with the use of Depo-Provera CI are discussed in greater detail in the Warnings and Precautions section (5):
- •
- Loss of Bone Mineral Density [see Warnings and Precautions (5.1)]
- •
- Thromboembolic disease [see Warnings and Precautions (5.2)]
- •
- Breast Cancer [see Warnings and Precautions (5.3)]
- •
- Anaphylaxis and Anaphylactoid Reactions [see Warnings and Precautions (5.5)]
- •
- Bleeding Irregularities [see Warnings and Precautions (5.10)]
- •
- Weight Gain [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Clinical trials are conducted under widely varying conditions, therefore, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the two clinical trials with Depo-Provera CI, over 3,900 women, who were treated for up to 7 years, reported the following adverse reactions, which may or may not be related to the use of Depo-Provera CI. The population studied ranges in age from 15 to 51 years, of which 46% were White, 50% Non-White, and 4.9% Unknown race. The patients received 150 mg Depo-Provera CI every 3-months (90 days). The median study duration was 13 months with a range of 1-84 months. Fifty‑eight percent of patients remained in the study after 13 months and 34% after 24 months.
Table 1. Adverse Reactions that Were Reported by More than 5% of Subjects
|
|
|
|
|
|
|
|
|
|
- * Body System represented from COSTART medical dictionary.
- Table 2. Adverse Reactions that Were Reported by between 1 and 5% of Subjects
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- * Body System represented from COSTART medical dictionary.
Adverse reactions leading to study discontinuation in ≥2% of subjects: bleeding (8.2%), amenorrhea (2.1%), weight gain (2.0%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Depo-Provera CI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been cases of osteoporosis including osteoporotic fractures reported post-marketing in patients taking Depo-Provera CI.
- Table 3. Adverse Reactions Reported during Post-Marketing Experience
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- * Body System represented from COSTART medical dictionary.
- † Injection site abscess and injection site infections have been reported; therefore, strict aseptic injection technique should be followed when administering Depo‑Provera CI in order to avoid injection site infections [see Dosage andAdministration (2.1)].
Related/similar drugs
7. Drug Interactions
7.1 Changes in Contraceptive Effectiveness Associated with Co-Administration of Other Products
If a woman on hormonal contraceptives takes a drug or herbal product that induces enzymes, including CYP3A4, that metabolize contraceptive hormones, counsel her to use additional contraception or a different method of contraception. Drugs or herbal products that induce such enzymes may decrease the plasma concentrations of contraceptive hormones, and may decrease the effectiveness of hormonal contraceptives. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include:
- •
- barbiturates
- •
- bosentan
- •
- carbamazepine
- •
- felbamate
- •
- griseofulvin
- •
- oxcarbazepine
- •
- phenytoin
- •
- rifampin
- •
- St. John's wort
- •
- topiramate
HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant changes (increase or decrease) in the plasma levels of progestin have been noted in some cases of co-administration of HIV protease inhibitors. Significant changes (increase or decrease) in the plasma levels of the progestin have been noted in some cases of co-administration with non-nucleoside reverse transcriptase inhibitors.
Antibiotics: There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
7.2 Laboratory Test Interactions
The pathologist should be advised of progestin therapy when relevant specimens are submitted.
The following laboratory tests may be affected by progestins including Depo-Provera CI:
(a) Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
(b) Gonadotropin levels are decreased.
(c) Sex-hormone-binding-globulin concentrations are decreased.
(d) Protein-bound iodine and butanol extractable protein-bound iodine may increase. T3-uptake values may decrease.
(e) Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
(f) Sulfobromophthalein and other liver function test values may be increased.
(g) The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There is no use for contraception in pregnancy; therefore, Depo-Provera CI should be discontinued during pregnancy.
Epidemiologic studies and meta‑analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to progestins before conception or during early pregnancy.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
Although medroxyprogesterone acetate is detectable in the milk of mothers receiving Depo-Provera CI, milk composition, quality, and amount do not appear to be adversely affected. Effects on milk production and lactation initiation/duration remain unclear when administered before 6 weeks after delivery, therefore, in mothers who exclusively breastfeed, initiate Depo-Provera CI during or after the sixth post-partum week [see Dosage and Administration (2.1)].
No adverse effects in breastfed infants would be expected with maternal use of progestins. Neonates and infants exposed to medroxyprogesterone acetate from breast milk have been studied and no adverse effects have been noted.
The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for Depo-Provera CI and any potential adverse effects on the breastfed child from Depo-Provera CI or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Depo-Provera CI is indicated for the prevention of pregnancy and would therefore be expected to impair female fertility until cessation of treatment. Women may experience a delay in return to ovulation and fertility (conception) following discontinuation of Depo-Provera CI [see Warnings and Precautions (5.14)].
8.4 Pediatric Use
Depo-Provera CI is not indicated before menarche. Use of Depo-Provera CI is associated with significant loss of BMD. This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity. It is unknown if use of Depo-Provera CI by younger women will reduce peak bone mass and increase the risk of osteoporotic fractures in later life. Other than concerns about loss of BMD, the safety and effectiveness are expected to be the same for postmenarchal adolescents and adult women.
11. Depo-Provera Contraceptive Description
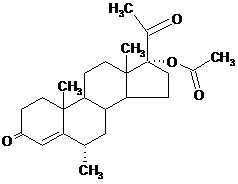
Depo-Provera CI contains medroxyprogesterone acetate, a derivative of progesterone, as its active ingredient. Medroxyprogesterone acetate is active by the parenteral and oral routes of administration. It is a white to off‑white; odorless crystalline powder that is stable in air and that melts between 200°C and 210°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and insoluble in water.
The chemical name for medroxyprogesterone acetate is pregn-4-ene-3, 20-dione, 17-(acetyloxy)-6-methyl-, (6α-).
The structural formula is as follows:
Depo-Provera CI for IM injection is available in vials and prefilled syringes, each containing 1 mL of medroxyprogesterone acetate sterile aqueous suspension 150 mg/mL.
|
For Depo-Provera CI vials, each mL of sterile aqueous suspension contains: |
|
|
Medroxyprogesterone acetate |
150 mg |
|
Polyethylene glycol 3350 |
28.9 mg |
|
Polysorbate 80 |
2.41 mg |
|
Sodium chloride |
8.68 mg |
|
Methylparaben |
1.37 mg |
|
Propylparaben |
0.150 mg |
|
Water for injection |
quantity sufficient |
|
When necessary, pH is adjusted with sodium hydroxide or hydrochloric acid, or both. |
|
|
For Depo-Provera CI prefilled syringes, each mL of sterile aqueous suspension contains: |
|
|
Medroxyprogesterone acetate |
150 mg |
|
Polyethylene glycol 3350 |
28.5 mg |
|
Polysorbate 80 |
2.37 mg |
|
Sodium chloride |
8.56 mg |
|
Methylparaben |
1.35 mg |
|
Propylparaben |
0.147 mg |
|
Water for injection |
quantity sufficient |
|
When necessary, pH is adjusted with sodium hydroxide or hydrochloric acid, or both. |
|
12. Depo-Provera Contraceptive - Clinical Pharmacology
12.1 Mechanism of Action
Depo-Provera CI (medroxyprogesterone acetate [MPA]) inhibits the secretion of gonadotropins which primarily prevents follicular maturation and ovulation and causes thickening of cervical mucus. These actions contribute to its contraceptive effect.
12.3 Pharmacokinetics
Absorption
Following a single 150 mg IM dose of Depo-Provera CI in eight women between the ages of 28 and 36 years old, medroxyprogesterone acetate concentrations, measured by an extracted radioimmunoassay procedure, increase for approximately 3 weeks to reach peak plasma concentrations of 1 to 7 ng/mL.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin. No binding of MPA occurs with sex-hormone-binding globulin (SHBG).
Elimination
Metabolism
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Excretion
The concentrations of medroxyprogesterone acetate decrease exponentially until they become undetectable (<100 pg/mL) between 120 to 200 days following injection. Using an unextracted radioimmunoassay procedure for the assay of medroxyprogesterone acetate in serum, the apparent half-life for medroxyprogesterone acetate following IM administration of Depo-Provera CI is approximately 50 days. Most medroxyprogesterone acetate metabolites are excreted in the urine as glucuronide conjugates with only minor amounts excreted as sulfates.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
[see Warnings and Precautions (5.3, 5.14) and Use in Specific Populations (8.3)].
14. Clinical Studies
14.1 Contraception
In five clinical studies using Depo-Provera CI, the 12-month failure rate for the group of women treated with Depo-Provera CI was zero (no pregnancies reported) to 0.7 by Life-Table method. The effectiveness of Depo‑Provera CI is dependent on the patient returning every 3 months (13 weeks) for reinjection.
14.2 Bone Mineral Density Changes in Women Treated with DepoProvera CI
In a controlled, clinical study, adult women using Depo-Provera CI (150mg) for up to 5 years showed spine and hip bone mineral density (BMD) mean decreases of 5–6%, compared to no significant change in BMD in the control group. The decline in BMD was more pronounced during the first two years of use, with smaller declines in subsequent years. Mean changes in lumbar spine BMD of ‑2.86%, ‑4.11%, ‑4.89%, ‑4.93% and ‑5.38% after 1, 2, 3, 4, and 5 years, respectively, were observed. Mean decreases in BMD of the total hip and femoral neck were similar.
After stopping use of Depo-Provera CI, there was partial recovery of BMD toward baseline values during the 2-year post-therapy period. Longer duration of treatment was associated with less complete recovery during this 2-year period following the last injection. Table 4 shows the change in BMD in women after 5 years of treatment with Depo-Provera CI and in women in a control group, as well as the extent of recovery of BMD for the subset of the women for whom 2-year post treatment data were available.
| Time in Study | Spine | Total Hip | Femoral Neck | |||
|---|---|---|---|---|---|---|
| Depo-Provera* | Control† | Depo-Provera* | Control† | Depo-Provera* | Control† | |
|
5 years |
-5.38% |
0.43% |
-5.16% |
0.19% |
-6.12% |
-0.27% |
|
7 years |
-3.13% |
0.53% |
-1.34% |
0.94% |
-5.38% |
-0.11% |
14.3 Bone Mineral Density Changes in Adolescent Females (12 to 18 Years of Age) Treated with DepoProvera CI
The impact of Depo-Provera CI (150 mg) use for up to 240 weeks (4.6 years) was evaluated in an open-label non-randomized clinical study in 389 adolescent females (12 to 18 years of age). Use of Depo-Provera CI was associated with a significant decline from baseline in BMD.
Partway through the trial, drug administration was stopped (at 120 weeks). The mean number of injections per Depo-Provera CI user was 9.3. Table 5 summarizes the study findings. The decline in BMD at total hip and femoral neck was greater with longer duration of use. The mean decrease in BMD at 240 weeks was more pronounced at total hip (-6.4%) and femoral neck (-5.4%) compared to lumbar spine (-2.1%).
Adolescents in the untreated cohort had an increase in BMD during the period of growth following menarche. However, the two cohorts were not matched at baseline for age, gynecologic age, race, BMD and other factors that influence the rate of acquisition of BMD.
| Duration of Treatment | Depo-Provera CI
(150 mg IM) | Unmatched, Untreated Cohort | ||
|---|---|---|---|---|
| N | Mean % Change | N | Mean % Change | |
|
Total Hip BMD | ||||
|
Week 60 (1.2 years) |
113 |
-2.75 |
166 |
1.22 |
|
Week 120 (2.3 years) |
73 |
-5.40 |
109 |
2.19 |
|
Week 240 (4.6 years) |
28 |
-6.40 |
84 |
1.71 |
|
Femoral Neck BMD | ||||
|
Week 60 |
113 |
-2.96 |
166 |
1.75 |
|
Week 120 |
73 |
-5.30 |
108 |
2.83 |
|
Week 240 |
28 |
-5.40 |
84 |
1.94 |
|
Lumbar Spine BMD | ||||
|
Week 60 |
114 |
-2.47 |
167 |
3.39 |
|
Week 120 |
73 |
-2.74 |
109 |
5.28 |
|
Week 240 |
27 |
-2.11 |
84 |
6.40 |
BMD Recovery Post-Treatment in Adolescents
Longer duration of treatment and smoking were associated with less recovery of BMD following the last injection of Depo-Provera CI. Table 6 shows the extent of recovery of BMD up to 60 months post-treatment for adolescents who received Depo-Provera CI for two years or less compared to more than two years. Post‑treatment follow-up showed that, in women treated for more than two years, only lumbar spine BMD recovered to baseline levels after treatment was discontinued. Adolescents treated with Depo-Provera CI for more than two years did not recover to their baseline BMD level at femoral neck and total hip even up to 60 months post-treatment. Adolescents in the untreated cohort gained BMD throughout the trial period (data not shown) [see Warnings and Precautions (5.1)].
| Duration of Treatment | 2 years or less | More than 2 years | ||
|---|---|---|---|---|
| N | Mean % Change from baseline | N | Mean % Change from baseline | |
|
Total Hip BMD |
||||
|
End of Treatment |
49 |
-1.5% |
49 |
-6.2% |
|
12 M post-treatment |
33 |
-1.4% |
24 |
-4.6% |
|
24 M post-treatment |
18 |
0.3% |
17 |
-3.6% |
|
36 M post-treatment |
12 |
2.1% |
11 |
-4.6% |
|
48 M post-treatment |
10 |
1.3% |
9 |
-2.5% |
|
60 M post-treatment |
3 |
0.2% |
2 |
-1.0% |
|
Femoral Neck BMD |
||||
|
End of Treatment |
49 |
-1.6% |
49 |
-5.8% |
|
12 M post-treatment |
33 |
-1.4% |
24 |
-4.3% |
|
24 M post-treatment |
18 |
0.5% |
17 |
-3.8% |
|
36 M post-treatment |
12 |
1.2% |
11 |
-3.8% |
|
48 M post-treatment |
10 |
2.0% |
9 |
-1.7% |
|
60 M post-treatment |
3 |
1.0% |
2 |
-1.9% |
|
Lumbar Spine BMD |
||||
|
End of Treatment |
49 |
-0.9% |
49 |
-3.5% |
|
12 M post-treatment |
33 |
0.4% |
23 |
-1.1% |
|
24 M post-treatment |
18 |
2.6% |
17 |
1.9% |
|
36 M post-treatment |
12 |
2.4% |
11 |
0.6% |
|
48 M post-treatment |
10 |
6.5% |
9 |
3.5% |
|
60 M post-treatment |
3 |
6.2% |
2 |
5.7% |
14.4 Bone Fracture Incidence in Women Treated with DepoProvera CI
A retrospective cohort study to assess the association between Depo-Provera CI injection and the incidence of bone fractures was conducted in 312,395 female contraceptive users in the UK. The incidence rates of fracture were compared between Depo-Provera CI users and contraceptive users who had no recorded use of Depo-Provera CI. The Incident Rate Ratio (IRR) for any fracture during the follow-up period (mean=5.5 years) was 1.41 (95% CI 1.35, 1.47). It is not known if this is due to Depo-Provera CI use or to other related lifestyle factors that have a bearing on fracture rate.
In the study, when cumulative exposure to Depo-Provera CI was calculated, the fracture rate in users who received fewer than 8 injections was higher than that in women who received 8 or more injections. However, it is not clear that cumulative exposure, which may include periods of intermittent use separated by periods of non-use, is a useful measure of risk, as compared to exposure measures based on continuous use.
There were very few osteoporotic fractures (fracture sites known to be related to low BMD) in the study overall, and the incidence of osteoporotic fractures was not found to be higher in Depo-Provera CI users compared to non-users. Importantly, this study could not determine whether use of Depo-Provera CI has an effect on fracture rate later in life.
15. References
- 1.
- Li CI, Beaber EF, Tang, MCT et al. Effect of Depo-Medroxyprogesterone Acetate on Breast Cancer Risk among Women 20 to 44 years of Age. Cancer Research 2012;72:2028–2035.
- 2.
- Paul C, Skegg DCG, Spears GFS. Depot medroxyprogesterone (Depo-Provera) and risk of breast cancer. Br Med J 1989; 299:759–62.
16. How is Depo-Provera Contraceptive supplied
Depo-Provera CI is supplied in the following strengths and package configurations:
| Package Configuration | Strength | NDC |
|---|---|---|
|
Depo-Provera CI (medroxyprogesterone acetate sterile aqueous suspension 150 mg/mL) |
||
|
1 mL vial |
150 mg/mL |
NDC 0009-0746-30 |
|
25 × 1 mL vials |
150 mg/mL |
NDC 0009-0746-35 |
|
Depo-Provera CI prefilled syringes packaged with 22 gauge × 1 1/2 inch Terumo® SurGuard™ Needles |
||
|
1 mL prefilled syringe |
150 mg/mL |
NDC 0009-7376-11 |
17. Patient Counseling Information
Advise the patient to read the FDA‑approved patient labeling (Patient Information).
- •
- Advise patients at the beginning of treatment that their menstrual cycle may be disrupted and that irregular and unpredictable bleeding or spotting results, and that this usually decreases to the point of amenorrhea as treatment with Depo-Provera CI continues, without other therapy being required.
- •
- Counsel patients about the possible increased risk of breast cancer in women who use Depo-Provera CI [see Warnings and Precautions (5.3)].
- •
- Counsel patients that this product does not protect against HIV infection (AIDS) and other sexually transmitted infections.
- •
- Counsel patients on Warnings and Precautions associated with use of Depo-Provera CI.
- •
- Counsel patients to use a back-up method or alternative method of contraception when enzyme inducers are used with Depo-Provera CI.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
To contact Pfizer’s Medical Information Department please visit www.pfizermedinfo.com or call 1‑800‑438‑1985.
Patient Information
Depo-Provera® (DEP-po pro-VAIR-ah) CI
(medroxyprogesterone acetate injectable suspension)
Contraceptive Injection
Read this Patient Information carefully before you decide if Depo-Provera CI is right for you. This information does not take the place of talking with your gynecologist or other healthcare professional who specializes in women's health. If you have any questions about Depo-Provera CI, ask your healthcare professional. You should also learn about other birth control methods to choose the one that is best for you.
What is the most important information I should know about Depo-Provera CI?
Depo-Provera CI can cause serious side effects, including:
- •
- Use of Depo-Provera CI may cause you to lose calcium stored in your bone and decrease your bone mass. The longer you use Depo-Provera CI, the greater your loss of calcium from your bones. Your bones may not recover completely when you stop using Depo-Provera CI.
- •
- If you use Depo-Provera CI continuously for a long time (for more than 2 years), it may increase the risk of weak, porous bones (osteoporosis) that could increase the risk of broken bones, especially after menopause.
- •
- You should not use Depo-Provera CI for more than two years unless you cannot use other birth control methods.
- •
- It is not known if your risk of developing osteoporosis is greater if you are a teenager or young adult when you start to use Depo-Provera CI (see "What are the possible side effects of Depo-Provera CI?").
Depo-Provera CI is intended to prevent pregnancy. Depo-Provera CI does not protect against HIV infection (AIDS) and other sexually transmitted infections (STIs).
What is Depo-Provera CI?
Depo-Provera CI is a progestin hormone birth control method that is given by injection (a shot) to prevent pregnancy.
How well does Depo-Provera CI work?
Your chance of getting pregnant depends on how well you follow the directions for taking your Depo-Provera CI. The more carefully you follow the directions (such as returning every 3 months for your next injection), the less chance you have of getting pregnant.
In clinical studies, about 1 out of 100 women got pregnant during the first year that they used Depo-Provera CI.
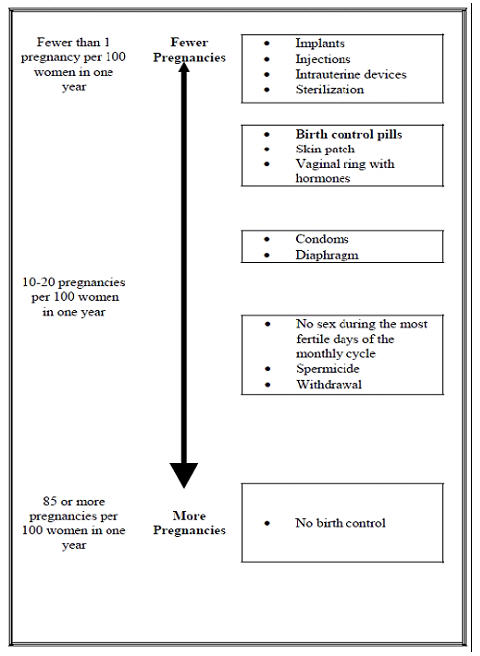
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
How should I take Depo-Provera CI?
- •
- Depo-Provera CI is given by your healthcare professional as a shot into your muscle (intramuscular injection). The shot is given in your buttock or upper arm 1 time every 3 months. At the end of the 3 months, you will need to return to your healthcare professional for your next injection in order to continue your protection against pregnancy.
- •
-
To make sure that you are not pregnant before you take Depo-Provera CI, the first injection should be given only:
- o
- during the first 5 days of a normal menstrual period, or
- o
- within the first 5 days after giving birth, if you are not breastfeeding, or
- o
- at the 6th week after giving birth, if you are feeding your baby only breastmilk.
- •
- Depo-Provera CI may be given at other times than those listed above, but you will likely need to have a pregnancy test first to show that you are not pregnant.
- •
- During treatment with Depo-Provera CI, you should see your healthcare professional every year for a blood pressure check and other healthcare needs.
Who Should Not Use Depo-Provera CI?
Do not use Depo-Provera CI if you:
- •
- have bleeding from your vagina that has not been explained
- •
- have breast cancer now or in the past, or think you have breast cancer
- •
- have had a stroke
- •
- ever had blood clots in your arms, legs or lungs
- •
- have problems with your liver or liver disease
- •
- are allergic to medroxyprogesterone acetate or any of the other ingredients in Depo-Provera CI. See the end of this leaflet for a complete list of ingredients in Depo-Provera CI.
What should I tell my healthcare professional before taking Depo-Provera CI?
Before taking Depo-Provera CI, tell your healthcare professional if you have:
- •
- risk factors for weak bones (osteoporosis) such as bone disease, use alcohol or smoke regularly, anorexia nervosa, or a strong family history of osteoporosis
- •
- irregular or lighter than usual menstrual periods
- •
- breast cancer now or in the past, or think you have breast cancer
- •
- a family history of breast cancer
- •
- an abnormal mammogram (breast X-ray), lumps in your breasts, or bleeding from your nipples
- •
- kidney problems
- •
- high blood pressure
- •
- had a stroke
- •
- had blood clots in your arms, legs or lungs
- •
- migraine headaches
- •
- asthma
- •
- epilepsy (convulsions or seizures)
- •
- diabetes
- •
- depression or a history of depression
- •
- any other medical conditions
If you are breastfeeding or plan to breastfeed, Depo-Provera CI can pass into your breast milk. Talk to your healthcare professional about the best way to feed your baby if you take Depo-Provera CI.
Tell your healthcare professional about all of the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Depo-Provera CI and certain other medicines may affect each other, causing serious side effects. Sometimes the doses of other medicines may need to be changed while you are taking Depo-Provera CI.
Some medicines may make Depo-Provera CI less effective at preventing pregnancy, including those listed below.
Especially tell your healthcare professional if you take:
- •
- medicine to help you sleep
- •
- bosentan
- •
- medicine for seizures
- •
- griseofulvin
- •
- an antibiotic
- •
- medicine for HIV (AIDS)
- •
- St. John's wort
Know the medicines you take. Keep a list of your medicines with you to show your healthcare professional or pharmacist before you first start taking Depo-Provera CI or when you get a new medicine.
Follow your healthcare professional's instructions about using a back-up method of birth control if you are taking medicines that may make Depo-Provera CI less effective.
What are the possible side effects of Depo-Provera CI?
Depo-Provera CI can cause serious side effects, including:
- •
- Effect on the bones: See "What is the most important information I should know about Depo-Provera CI?".
Teenage years are the most important years to gain bone strength. The decrease in calcium in your bones is of most concern if you are a teenager or have the following problems:- •
- bone disease
- •
- an eating disorder (anorexia nervosa)
- •
- a strong family history of osteoporosis
- •
- you take a drug that can lower the amount of calcium in your bones (drugs for epilepsy or steroid drugs)
- •
- you drink a lot of alcohol (more than 2 drinks a day)
- •
- you smoke
- If you need a birth control method for more than 2 years, your healthcare professional may switch you to another birth control method instead of using Depo-Provera CI. If you continue using Depo-Provera CI, your healthcare professional may ask you to have a bone test, especially if you have other risks for weak bones.
- When Depo-Provera CI is stopped, your bones may start to regain calcium. However, in a study of teenage girls who used Depo-Provera CI for more than 2 years, their hip bones did not completely recover by 5 years after they stopped using Depo-Provera CI. Taking calcium and Vitamin D and exercising daily may lessen the loss of calcium from your bones.
- •
- possible increased risk of breast cancer. Women who use Depo-Provera CI may have a slightly increased risk of breast cancer compared to non-users.
- •
- blood clots in your arms, legs, lungs, and eyes
- •
- stroke
- •
- a pregnancy outside of your uterus (ectopic pregnancy). Ectopic pregnancy is a medical emergency that often requires surgery. Ectopic pregnancy can cause internal bleeding, infertility, and even death.
- •
- allergic reactions. Severe allergic reactions have been reported in some women using Depo-Provera CI.
- •
- loss of vision or other eye problems
- •
- migraine headaches
- •
- depression
- •
- convulsions or seizures
- •
- liver problems
Call your healthcare professional right away if you have:
- •
- sharp chest pain, coughing up blood, or sudden shortness of breath (indicating a possible clot in the lung)
- •
- sudden severe headache or vomiting, dizziness or fainting, problems with your eyesight or speech, weakness, or numbness in an arm or leg (indicating a possible stroke)
- •
- severe pain or swelling in the calf (indicating a possible clot in the leg)
- •
- sudden blindness, partial or complete (indicating a possible clot in the blood vessels of the eye)
- •
- unusually heavy vaginal bleeding
- •
- severe pain or tenderness in the lower abdominal area
- •
- persistent pain, pus, or bleeding at the injection site
- •
- yellowing of the eyes or skin
- •
- hives
- •
- difficulty breathing
- •
- swelling of the face, mouth, tongue or neck
The most common side effects of Depo-Provera CI include:
- •
- irregular vaginal bleeding, such as lighter or heavier menstrual bleeding, or continued spotting
- •
- weight gain. You may experience weight gain while you are using Depo-Provera CI. About two-thirds of the women who used Depo-Provera CI in the clinical trials reported a weight gain of about 5 pounds during the first year of use. You may continue to gain weight after the first year. Women who used Depo-Provera CI for 2 years gained an average of 8 pounds over those 2 years.
- •
- abdominal pain
- •
- headache
- •
- weakness
- •
- tiredness
- •
- nervousness
- •
- dizziness
Tell your healthcare professional if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of Depo-Provera CI. For more information, ask your healthcare professional or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088.
What other information should I know before choosing Depo-Provera CI?
- •
- Pregnancy. When you take Depo-Provera CI every 3 months, your chance of getting pregnant is very low. You could miss a period or have a light period and not be pregnant. If you miss 1 or 2 periods and think you might be pregnant, see your healthcare professional as soon as possible. You should not use Depo-Provera CI if you are pregnant. However, Depo-Provera CI taken by accident during pregnancy does not seem to cause birth defects.
- •
- Nursing Mothers. Although Depo-Provera CI can be passed to the nursing baby in the breast milk, no harmful effects on babies have been found. Depo-Provera CI does not stop the breasts from producing milk, so it can be used by nursing mothers. However, to minimize the amount of Depo-Provera CI that is passed to the baby in the first weeks after birth, you should wait until your baby is 6 weeks old before you start using Depo-Provera CI for birth control.
How will Depo-Provera CI change my periods?
- •
-
Change in normal menstrual cycle. The side effect reported most frequently by women who use Depo-Provera CI for birth controls is a change in their normal menstrual cycle. During the first year of using Depo-Provera CI, you might have one or more of the following changes:
- o
- irregular or unpredictable bleeding or spotting
- o
- an increase or decrease in menstrual bleeding
- o
- no bleeding at all. In clinical studies of Depo-Provera CI, 55% of women reported no menstrual bleeding (amenorrhea) after one year of use and 68% of women reported no menstrual bleeding after two years of use.
- •
- Missed period. During the time you are using Depo-Provera CI for birth controls, you may skip a period, or your periods may stop completely. If you have been receiving your shot of Depo-Provera CI regularly every 3 months, then you are probably not pregnant. However, if you think that you may be pregnant, see your healthcare professional.
Unusually heavy or continuous bleeding is not a usual effect of Depo-Provera CI and if this happens you should see your healthcare professional right away.
With continued use of Depo-Provera CI, bleeding usually decreases and many women stop having periods completely. When you stop using Depo-Provera CI your menstrual period will usually, in time, return to its normal cycle.
What if I want to become pregnant?
Because Depo-Provera CI is a long-acting birth control method, it takes some time after your last shot for its effect to wear off. Most women who try to get pregnant after using Depo-Provera CI get pregnant within 18 months after their last shot. The length of time you use Depo-Provera CI has no effect on how long it takes you to become pregnant after you stop using it.
General Information about Depo-Provera CI
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. This leaflet summarizes the most important information about Depo-Provera CI. If you would like more information, talk with your healthcare professional. You can ask your healthcare professional for information about Depo-Provera CI that is written for healthcare professionals.
What are the ingredients in Depo-Provera CI?
Active ingredient: medroxyprogesterone acetate
Inactive ingredients: polyethylene glycol 3350, polysorbate 80, sodium chloride, methylparaben, propylparaben, and water for injection. When necessary, pH is adjusted with sodium hydroxide or hydrochloric acid, or both.
This Patient Information has been approved by the U.S. Food and Drug Administration.
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
LAB-0148-14.0
Revised July 2024
PRINCIPAL DISPLAY PANEL - 150 mg/mL Vial Label
NDC 0009-0746-30
Rx only
Depo-Provera®
Contraceptive
Injection
medroxyPROGESTERone acetate
injectable suspension, USP
150 mg/mL
PRINCIPAL DISPLAY PANEL - 150 mg/mL Vial Carton
Pfizer
NDC 0009-0746-30
Rx only
Depo-Provera®
Contraceptive
Injection
medroxyPROGESTERone
acetate injectable
suspension, USP
150 mg/mL
1 mL Single-Dose Vial
For intramuscular use only
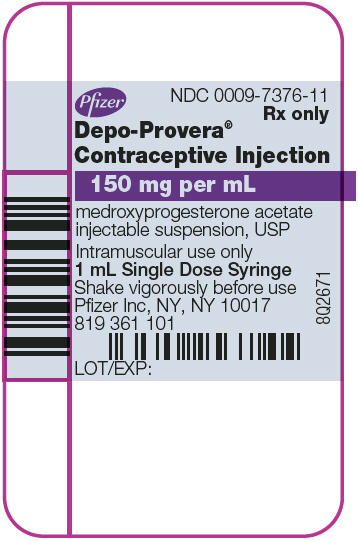
PRINCIPAL DISPLAY PANEL - 1 mL Syringe Label
Pfizer
NDC 0009-7376-11
Rx only
Depo-Provera®
Contraceptive Injection
150 mg per mL
medroxyprogesterone acetate
injectable suspension, USP
Intramuscular use only
1 mL Single Dose Syringe
Shake vigorously before use
Pfizer Inc, NY, NY 10017
819 361 101
LOT/EXP:
8Q2671
| DEPO-PROVERA
medroxyprogesterone acetate injection, suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| DEPO-PROVERA
medroxyprogesterone acetate injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-0746, 0009-7376) , MANUFACTURE(0009-0746, 0009-7376) , API MANUFACTURE(0009-0746, 0009-7376) , PACK(0009-0746, 0009-7376) , LABEL(0009-0746, 0009-7376) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | MANUFACTURE(0009-0746, 0009-7376) , PACK(0009-0746, 0009-7376, 0009-0746, 0009-7376) , LABEL(0009-0746, 0009-7376) | |
Frequently asked questions
- Is it normal to have discharge on Depo shot?
- What are my birth control options and how effective are they?
- My stool has changed color. What does it mean?
- How long after having a Depo Provera shot can you have unprotected sex?
- Why is my poop green? What does this mean?
More about Depo Provera (medroxyprogesterone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1,969)
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Support group
- Drug class: contraceptives
- Breastfeeding
- En español