Isocarboxazid
Medically reviewed by Drugs.com. Last updated on Sep 30, 2024.
Pronunciation
(eye soe kar BOKS a zid)
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
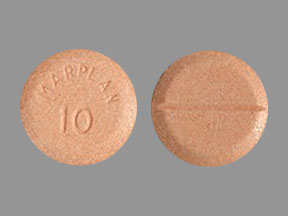
Marplan: 10 mg [scored]
Brand Names: U.S.
- Marplan
Pharmacologic Category
- Antidepressant, Monoamine Oxidase Inhibitor
Pharmacology
Thought to act by increasing endogenous concentrations of epinephrine, norepinephrine, dopamine, and serotonin through inhibition of the enzyme (monoamine oxidase) responsible for the breakdown of these neurotransmitters
Onset of Action
Depression: Initial effects may be observed within 1 to 2 weeks of treatment, with continued improvements through 4 to 6 weeks (Papakostas 2006; Posternak 2005; Szegedi 2009).
Use: Labeled Indications
Treatment of depression
Contraindications
Hypersensitivity to isocarboxazid or any component of the formulation; cardiovascular disease (including hypertension); cerebrovascular defect (suspected or confirmed); history of headache; history of hepatic disease or abnormal liver function tests; pheochromocytoma; severe renal impairment
Concurrent use of antihistamines, antihypertensives, bupropion, buspirone, caffeine (excessive use), CNS depressants (including ethanol and opioids), dextromethorphan, diuretics, elective surgery requiring general anesthesia (discontinue isocarboxazid ≥10 days prior to elective surgery), local vasoconstrictors, meperidine, MAO inhibitors or dibenzazepine derivatives (eg, amitriptyline, clomipramine, desipramine, imipramine, nortriptyline, protriptyline, doxepin, carbamazepine, cyclobenzaprine, amoxapine, maprotiline, trimipramine), SSRIs or SNRIs, spinal anesthesia (hypotension may be exaggerated), sympathomimetics (including amphetamines, cocaine, phenylephrine, pseudoephedrine) or related compounds (methyldopa, reserpine, levodopa, tryptophan), or foods high in tyramine content
Bupropion: At least 14 days should elapse between MAO inhibitor discontinuation and bupropion initiation.
Buspirone: At least 10 days should elapse between isocarboxazid discontinuation and buspirone initiation.
MAO inhibitors or dibenzazepine derivatives: At least 1 week should elapse between the use of another MAO inhibitor or dibenzazepine derivative and isocarboxazid use.
Meperidine: At least 2-3 weeks should elapse between MAO inhibitor discontinuation and meperidine use.
SSRIs or SNRIs: At least 2 weeks should elapse between the discontinuation of sertraline or paroxetine and the initiation of isocarboxazid. At least 5 weeks should elapse between the discontinuation of fluoxetine and the initiation of isocarboxazid. At least 1 week should elapse between discontinuation of a SNRI and the initiation of isocarboxazid. At least 2 weeks should elapse between the discontinuation of isocarboxazid and the initiation of SSRIs.
Dosing: Adult
Depression: Oral: Initial: 10 mg 2 times/day; may increase by 10 mg/day every 2 to 4 days to 40 mg/day by the end of the first week (divided into 2 to 4 doses). After first week, may increase by up to 20 mg/week to a maximum of 60 mg/day. May take 3 to 6 weeks to see effects. Dose should be reduced once maximum clinical effect is seen. If no response obtained within 6 weeks, additional titration is unlikely to be beneficial. Note: Use caution in patients on >40 mg/day; experience is limited.
Discontinuation of therapy: When discontinuing antidepressant treatment that has lasted for >3 weeks, gradually taper the dose (eg, over 2 to 4 weeks) to minimize withdrawal symptoms and detect reemerging symptoms (APA 2010; WFSBP [Bauer 2015]). Reasons for a slower titration (eg, over 4 weeks) include use of a drug with a half-life <24 hours (eg, paroxetine, venlafaxine), prior history of antidepressant withdrawal symptoms, or high doses of antidepressants (APA 2010; Hirsch 2019). More severe symptoms have been associated with monoamine oxidase (MAO) inhibitors; more conservative tapers may be necessary (Haddad 2001; Shelton 2001). If intolerable withdrawal symptoms occur, resume the previously prescribed dose and/or decrease dose at a more gradual rate (Shelton 2001). Select patients (eg, those with a history of discontinuation syndrome) on long-term treatment (>6 months) may benefit from tapering over >3 months (WFSBP [Bauer 2015]). Evidence supporting ideal taper rates is limited (Shelton 2001; WFSBP [Bauer 2015]).
MAO inhibitor recommendations:
Switching to or from an MAO inhibitor intended to treat psychiatric disorders:
Allow 14 days to elapse between discontinuing an alternative antidepressant without long half-life metabolites (eg, TCAs, paroxetine, fluvoxamine, venlafaxine) or MAO inhibitor intended to treat psychiatric disorders and initiation of isocarboxazid.
Allow 5 weeks to elapse between discontinuing fluoxetine (long half-life metabolites) intended to treat psychiatric disorders and initiation of isocarboxazid.
Allow at least 7 to 14 days to elapse between discontinuing isocarboxazid and initiation of an alternative antidepressant or MAO inhibitor intended to treat psychiatric disorders.
Use with other MAO inhibitors (such as linezolid or IV methylene blue):
Do not initiate isocarboxazid in patients receiving linezolid or IV methylene blue; consider other interventions for psychiatric condition.
If urgent treatment with linezolid or IV methylene blue is required in a patient already receiving isocarboxazid and potential benefits outweigh potential risks, discontinue isocarboxazid promptly and administer linezolid or IV methylene blue. Monitor for serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or IV methylene blue, whichever comes first. May resume isocarboxazid 24 hours after the last dose of linezolid or IV methylene blue.
Dosing: Geriatric
Refer to adult dosing.
Dietary Considerations
Avoid tyramine-containing foods/beverages. Some examples include aged or matured cheese, air-dried or cured meats (including sausages and salamis), fava or broad bean pods, tap/draft beers, Marmite concentrate, sauerkraut, soy sauce and other soybean condiments. Food’s freshness is also an important concern; improperly stored or spoiled food can create an environment where tyramine concentrations may increase.
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect from light.
Drug Interactions
Acetylcholinesterase Inhibitors: May diminish the therapeutic effect of Anticholinergic Agents. Anticholinergic Agents may diminish the therapeutic effect of Acetylcholinesterase Inhibitors. Monitor therapy
Aclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Agents with Blood Glucose Lowering Effects: Monoamine Oxidase Inhibitors may enhance the hypoglycemic effect of Agents with Blood Glucose Lowering Effects. Monitor therapy
Alcohol (Ethyl): May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Alosetron: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Alpha-/Beta-Agonists (Indirect-Acting): Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Alpha-/Beta-Agonists (Indirect-Acting). While linezolid is expected to interact via this mechanism, management recommendations differ from other monoamine oxidase inhibitors. Refer to linezolid specific monographs for details. Avoid combination
Alpha1-Agonists: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Alpha1-Agonists. While linezolid is expected to interact via this mechanism, management recommendations differ from other monoamine oxidase inhibitors. Refer to linezolid specific monographs for details. Avoid combination
Altretamine: May enhance the orthostatic hypotensive effect of Monoamine Oxidase Inhibitors (Antidepressant). Monitor therapy
Amantadine: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Amifampridine: Agents With Seizure Threshold Lowering Potential may enhance the neuroexcitatory and/or seizure-potentiating effect of Amifampridine. Monitor therapy
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When used at chemotherapy doses, hold blood pressure lowering medications for 24 hours before amifostine administration. If blood pressure lowering therapy cannot be held, do not administer amifostine. Use caution with radiotherapy doses of amifostine. Consider therapy modification
Amisulpride (Oral): May enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Amphetamines: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Amphetamines. While linezolid and tedizolid may interact via this mechanism, management recommendations differ from other monoamine oxidase inhibitors. Refer to monographs specific to those agents for details. Avoid combination
Anticholinergic Agents: May enhance the adverse/toxic effect of other Anticholinergic Agents. Monitor therapy
Antiemetics (5HT3 Antagonists): May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Antipsychotic Agents: Serotonergic Agents (High Risk) may enhance the adverse/toxic effect of Antipsychotic Agents. Specifically, serotonergic agents may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotic Agents may enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Apraclonidine: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Apraclonidine. Monoamine Oxidase Inhibitors may increase the serum concentration of Apraclonidine. Avoid combination
AtoMOXetine: Monoamine Oxidase Inhibitors may enhance the neurotoxic (central) effect of AtoMOXetine. Avoid combination
Atropine (Ophthalmic): Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Atropine (Ophthalmic). Avoid combination
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benzhydrocodone: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Management: The use of benzhydrocodone is not recommended for patients taking monoamine oxidase inhibitors (MAOIs) or within 14 days of MAOI discontinuation. If coadministration is required, use test doses and frequent titration of small benzhydrocodone. Consider therapy modification
Beta2-Agonists: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Beta2-Agonists. Monitor therapy
Betahistine: Monoamine Oxidase Inhibitors may increase the serum concentration of Betahistine. Monitor therapy
Bezafibrate: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Bezafibrate. Avoid combination
Blood Pressure Lowering Agents: May enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Botulinum Toxin-Containing Products: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Brexanolone: Isocarboxazid may enhance the CNS depressant effect of Brexanolone. Monitor therapy
Brimonidine (Ophthalmic): Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Brimonidine (Ophthalmic). Monoamine Oxidase Inhibitors may increase the serum concentration of Brimonidine (Ophthalmic). Monitor therapy
Brimonidine (Topical): Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Brimonidine (Topical). Monoamine Oxidase Inhibitors may increase the serum concentration of Brimonidine (Topical). Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Buprenorphine: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
BuPROPion: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of BuPROPion. Avoid combination
BusPIRone: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Cannabinoid-Containing Products: Anticholinergic Agents may enhance the tachycardic effect of Cannabinoid-Containing Products. Monitor therapy
CarBAMazepine: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Management: Avoid concurrent use of carbamazepine during, or within 14 days of discontinuing, treatment with a monoamine oxidase inhibitor. Avoid combination
Cerebrolysin: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Monitor therapy
Chloral Betaine: May enhance the adverse/toxic effect of Anticholinergic Agents. Monitor therapy
Chlorphenesin Carbamate: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Monitor therapy
Cimetropium: Anticholinergic Agents may enhance the anticholinergic effect of Cimetropium. Avoid combination
Clemastine: Monoamine Oxidase Inhibitors may enhance the anticholinergic effect of Clemastine. Monitor therapy
CloZAPine: Anticholinergic Agents may enhance the constipating effect of CloZAPine. Management: Consider alternatives to this combination whenever possible. If combined, monitor closely for signs and symptoms of gastrointestinal hypomotility and consider prophylactic laxative treatment. Consider therapy modification
Cocaine (Topical): May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Monitor therapy
Codeine: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Codeine. Avoid combination
COMT Inhibitors: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Management: Avoid coadministration of COMT inhibitors and nonselective monoamine oxidase inhibitors (MAOIs) (eg, isocarboxazid, phenelzine, tranylcypromine, linezolid, methylene blue) whenever possible. Consider therapy modification
Cyclobenzaprine: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Avoid combination
Cyproheptadine: Monoamine Oxidase Inhibitors may enhance the anticholinergic effect of Cyproheptadine. Cyproheptadine may diminish the serotonergic effect of Monoamine Oxidase Inhibitors. Avoid combination
Dapoxetine: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Do not use serotonergic agents (high risk) with dapoxetine or within 7 days of serotonergic agent discontinuation. Do not use dapoxetine within 14 days of monoamine oxidase inhibitor use. Dapoxetine labeling lists this combination as contraindicated. Avoid combination
Deutetrabenazine: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Deutetrabenazine. Avoid combination
Dexmethylphenidate: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Dexmethylphenidate. Avoid combination
Dextromethorphan: Monoamine Oxidase Inhibitors may enhance the serotonergic effect of Dextromethorphan. This may cause serotonin syndrome. Avoid combination
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Diethylpropion: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Diethylpropion. Avoid combination
Dihydrocodeine: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Avoid combination
Diphenoxylate: May enhance the hypertensive effect of Monoamine Oxidase Inhibitors. Avoid combination
Domperidone: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Domperidone. Monoamine Oxidase Inhibitors may diminish the therapeutic effect of Domperidone. Domperidone may diminish the therapeutic effect of Monoamine Oxidase Inhibitors. Monitor therapy
DOPamine: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of DOPamine. Management: Initiate dopamine at no greater than one-tenth (1/10) of the usual dose in patients who are taking (or have taken within the last 2 to 3 weeks) monoamine oxidase inhibitors. Monitor for an exaggerated hypertensive response to dopamine. Consider therapy modification
Doxapram: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Doxapram. Monitor therapy
Doxylamine: Monoamine Oxidase Inhibitors may enhance the anticholinergic effect of Doxylamine. Management: The US manufacturer of Diclegis (doxylamine/pyridoxine) and the manufacturers of Canadian doxylamine products specifically lists use with monoamine oxidase inhibitors as contraindicated. Monitor therapy
Droxidopa: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Droxidopa. Avoid combination
Eluxadoline: Anticholinergic Agents may enhance the constipating effect of Eluxadoline. Avoid combination
EPINEPHrine (Nasal): Monoamine Oxidase Inhibitors may enhance the hypertensive effect of EPINEPHrine (Nasal). Monitor therapy
EPINEPHrine (Oral Inhalation): Monoamine Oxidase Inhibitors may enhance the hypertensive effect of EPINEPHrine (Oral Inhalation). Avoid combination
Epinephrine (Racemic): Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Epinephrine (Racemic). Monitor therapy
EPINEPHrine (Systemic): Monoamine Oxidase Inhibitors may enhance the hypertensive effect of EPINEPHrine (Systemic). Monitor therapy
Ergot Derivatives: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Esketamine: May enhance the hypertensive effect of Monoamine Oxidase Inhibitors. Monitor therapy
Fenfluramine: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
FentaNYL: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Gastrointestinal Agents (Prokinetic): Anticholinergic Agents may diminish the therapeutic effect of Gastrointestinal Agents (Prokinetic). Monitor therapy
Glucagon: Anticholinergic Agents may enhance the adverse/toxic effect of Glucagon. Specifically, the risk of gastrointestinal adverse effects may be increased. Monitor therapy
Glycopyrrolate (Oral Inhalation): Anticholinergic Agents may enhance the anticholinergic effect of Glycopyrrolate (Oral Inhalation). Avoid combination
Glycopyrronium (Topical): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Guanethidine: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Heroin: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Heroin. Avoid combination
HYDROcodone: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of HYDROcodone. Management: Consider alternatives to this combination when possible. Consider therapy modification
HYDROmorphone: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of HYDROmorphone. Avoid combination
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Indoramin: Monoamine Oxidase Inhibitors may enhance the hypotensive effect of Indoramin. Avoid combination
Iobenguane Radiopharmaceutical Products: Monoamine Oxidase Inhibitors may diminish the therapeutic effect of Iobenguane Radiopharmaceutical Products. Management: Discontinue all drugs that may inhibit or interfere with catecholamine transport or uptake for at least 5 biological half-lives before iobenguane administration. Do not administer these drugs until at least 7 days after each iobenguane dose. Avoid combination
Iohexol: Agents With Seizure Threshold Lowering Potential may enhance the adverse/toxic effect of Iohexol. Specifically, the risk for seizures may be increased. Management: Discontinue agents that may lower the seizure threshold 48 hours prior to intrathecal use of iohexol. Wait at least 24 hours after the procedure to resume such agents. In nonelective procedures, consider use of prophylactic anticonvulsants. Consider therapy modification
Iomeprol: Agents With Seizure Threshold Lowering Potential may enhance the adverse/toxic effect of Iomeprol. Specifically, the risk for seizures may be increased. Management: Discontinue agents that may lower the seizure threshold 48 hours prior to intrathecal use of iomeprol. Wait at least 24 hours after the procedure to resume such agents. In nonelective procedures, consider use of prophylactic anticonvulsants. Consider therapy modification
Iopamidol: Agents With Seizure Threshold Lowering Potential may enhance the adverse/toxic effect of Iopamidol. Specifically, the risk for seizures may be increased. Management: Discontinue agents that may lower the seizure threshold 48 hours prior to intrathecal use of iopamidol. Wait at least 24 hours after the procedure to resume such agents. In nonelective procedures, consider use of prophylactic anticonvulsants. Consider therapy modification
Ipratropium (Oral Inhalation): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Isometheptene: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Isometheptene. Avoid combination
Itopride: Anticholinergic Agents may diminish the therapeutic effect of Itopride. Monitor therapy
Lasmiditan: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Levodopa-Containing Products: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Of particular concern is the development of hypertensive reactions when levodopa is used with nonselective MAOI. Avoid combination
Levomethadone: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Levonordefrin: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Levonordefrin. Avoid combination
Levosulpiride: Anticholinergic Agents may diminish the therapeutic effect of Levosulpiride. Avoid combination
Linezolid: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Lithium: Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of Lithium. This could result in serotonin syndrome. Management: Consider alternatives to this drug combination. If combined, monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes). Consider therapy modification
Lorcaserin (Withdrawn From US Market): May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Maprotiline: May enhance the hypertensive effect of Monoamine Oxidase Inhibitors. Avoid combination
Meperidine: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Meptazinol: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Meptazinol. Avoid combination
Mequitazine: Monoamine Oxidase Inhibitors may enhance the anticholinergic effect of Mequitazine. Avoid combination
Metaraminol: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Metaraminol. Monitor therapy
Metaxalone: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Methadone: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Avoid combination
Methyldopa: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Methyldopa. Avoid combination
Methylene Blue: Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of Methylene Blue. This could result in serotonin syndrome. Avoid combination
Methylphenidate: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Methylphenidate. Avoid combination
Metoclopramide: May enhance the hypertensive effect of Monoamine Oxidase Inhibitors. Avoid combination
Mianserin: Monoamine Oxidase Inhibitors may enhance the neurotoxic effect of Mianserin. Avoid combination
Mirabegron: Anticholinergic Agents may enhance the adverse/toxic effect of Mirabegron. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Monoamine Oxidase Inhibitors (Antidepressant): May enhance the hypertensive effect of other Monoamine Oxidase Inhibitors (Antidepressant). Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of other Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Monoamine Oxidase Inhibitors (Type B): Monoamine Oxidase Inhibitors (Antidepressant) may enhance the hypertensive effect of Monoamine Oxidase Inhibitors (Type B). Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Type B). This could result in serotonin syndrome. Avoid combination
Morphine (Systemic): Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Morphine (Systemic). Avoid combination
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nefazodone: Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of Nefazodone. This could result in serotonin syndrome. Avoid combination
Nefopam: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Nefopam. Avoid combination
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroglycerin: Anticholinergic Agents may decrease the absorption of Nitroglycerin. Specifically, anticholinergic agents may decrease the dissolution of sublingual nitroglycerin tablets, possibly impairing or slowing nitroglycerin absorption. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Norepinephrine: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Norepinephrine. Monitor therapy
Normethadone: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Normethadone. Avoid combination
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Ondansetron: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Opicapone: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Opioid Agonists: Anticholinergic Agents may enhance the adverse/toxic effect of Opioid Agonists. Specifically, the risk for constipation and urinary retention may be increased with this combination. Monitor therapy
Opioid Agonists: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Opium: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Opium. Avoid combination
Oxatomide: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Oxitriptan: Serotonergic Agents (High Risk) may enhance the serotonergic effect of Oxitriptan. This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
OxyCODONE: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Avoid combination
OxyMORphone: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Ozanimod: May enhance the hypertensive effect of Monoamine Oxidase Inhibitors. Avoid combination
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pheniramine: May enhance the anticholinergic effect of Monoamine Oxidase Inhibitors. Avoid combination
Pholcodine: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Avoid combination
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pizotifen: Monoamine Oxidase Inhibitors may enhance the anticholinergic effect of Pizotifen. Avoid combination
Potassium Chloride: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Chloride. Management: Patients on drugs with substantial anticholinergic effects should avoid using any solid oral dosage form of potassium chloride. Avoid combination
Potassium Citrate: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Citrate. Avoid combination
Pramlintide: May enhance the anticholinergic effect of Anticholinergic Agents. These effects are specific to the GI tract. Avoid combination
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Ramosetron: Anticholinergic Agents may enhance the constipating effect of Ramosetron. Monitor therapy
Ramosetron: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Reboxetine: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Reboxetine. Avoid combination
Remifentanil: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Remifentanil. Specifically, the risk for opioid toxicity (eg, respiratory depression) may be increased. Remifentanil may enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Management: The use of remifentanil is not recommended for patients taking monoamine oxidase inhibitors (MAOIs) or within 14 days of MAOI discontinuation. If coadministration is required, use test doses and titrate small doses of remifentanil frequently. Consider therapy modification
Reserpine: Monoamine Oxidase Inhibitors may enhance the adverse/toxic effect of Reserpine. Existing MAOI therapy can result in paradoxical effects of added reserpine (e.g., excitation, hypertension). Management: Monoamine oxidase inhibitors (MAOIs) should be avoided or used with great caution in patients who are also receiving reserpine. Monitor closely for paradoxical effects of reserpine (eg, excitation, hypertension). Consider therapy modification
Revefenacin: Anticholinergic Agents may enhance the anticholinergic effect of Revefenacin. Avoid combination
Secretin: Anticholinergic Agents may diminish the therapeutic effect of Secretin. Management: Avoid concomitant use of anticholinergic agents and secretin. Discontinue anticholinergic agents at least 5 half-lives prior to administration of secretin. Consider therapy modification
Selective Serotonin Reuptake Inhibitors: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Serotonergic Non-Opioid CNS Depressants: May enhance the serotonergic effect of Monoamine Oxidase Inhibitors (Antidepressant). This could result in serotonin syndrome. Avoid combination
Serotonin 5-HT1D Receptor Agonists (Triptans): May enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Monoamine Oxidase Inhibitors may increase the serum concentration of Serotonin 5-HT1D Receptor Agonists (Triptans). Avoid combination
Serotonin/Norepinephrine Reuptake Inhibitors: Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of Serotonin/Norepinephrine Reuptake Inhibitors. This could result in serotonin syndrome. Avoid combination
Solriamfetol: Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Solriamfetol. Avoid combination
St John's Wort: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. St John's Wort may decrease the serum concentration of Serotonergic Agents (High Risk). Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
SUFentanil: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Specifically, the risk for serotonin syndrome or opioid toxicities (eg, respiratory depression, coma) may be increased. Management: Sufentanil should not be used with monoamine oxidase (MAO) inhibitors (or within 14 days of stopping an MAO inhibitor) due to the potential for serotonin syndrome and/or excessive CNS depression. Avoid combination
Syrian Rue: May enhance the serotonergic effect of Serotonergic Agents (High Risk). This could result in serotonin syndrome. Management: Monitor for signs and symptoms of serotonin syndrome/serotonin toxicity (eg, hyperreflexia, clonus, hyperthermia, diaphoresis, tremor, autonomic instability, mental status changes) when these agents are combined. Monitor therapy
Tapentadol: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Specifically, the additive effects of norepinephrine may lead to adverse cardiovascular effects. Tapentadol may enhance the serotonergic effect of Monoamine Oxidase Inhibitors. This could result in serotonin syndrome. Avoid combination
Tetrabenazine: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Tetrahydrozoline (Nasal): Monoamine Oxidase Inhibitors may enhance the hypertensive effect of Tetrahydrozoline (Nasal). Avoid combination
Thiazide and Thiazide-Like Diuretics: Anticholinergic Agents may increase the serum concentration of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Tianeptine: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Tiotropium: Anticholinergic Agents may enhance the anticholinergic effect of Tiotropium. Avoid combination
Topiramate: Anticholinergic Agents may enhance the adverse/toxic effect of Topiramate. Monitor therapy
TraMADol: Monoamine Oxidase Inhibitors (Antidepressant) may enhance the adverse/toxic effect of TraMADol. Specifically, the risk for serotonin syndrome/serotonin toxicity and seizures may be increased.. Avoid combination
Tricyclic Antidepressants: Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of Tricyclic Antidepressants. This could result in serotonin syndrome. Avoid combination
Tryptophan: Monoamine Oxidase Inhibitors (Antidepressant) may enhance the serotonergic effect of Tryptophan. This could result in serotonin syndrome. Avoid combination
Umeclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Valbenazine: May enhance the adverse/toxic effect of Monoamine Oxidase Inhibitors. Avoid combination
Adverse Reactions
The following adverse drug reactions and incidences are derived from product labeling unless otherwise specified.
>10%: Central nervous system: Dizziness (29%), headache (15%)
1% to 10%:
Cardiovascular: Orthostatic hypotension (4%), palpitations (2%), syncope (2%)
Central nervous system: Disturbed sleep (5%), drowsiness (4%), anxiety (2%), chills (2%), feeling of heaviness (2%), forgetfulness (2%), hyperactivity (2%), lethargy (2%), myoclonus (2%), paresthesia (2%), sedation (2%)
Dermatologic: Diaphoresis (2%)
Gastrointestinal: Xerostomia (9%), constipation (7%), nausea (6%), diarrhea (2%)
Genitourinary: Urinary frequency (2%), impotence (2%), urinary hesitancy (1%)
Neuromuscular & skeletal: Tremor (4%)
<1%, postmarketing, and/or case reports: Akathisia, ataxia, coma, dysuria, euphoria, hallucination, hematologic abnormality, melanoglossia, neuritis, sexual disorder, SIADH (syndrome of inappropriate antidiuretic hormone secretion), skin photosensitivity, spider telangiectasia, toxic amblyopia, urinary incontinence, urinary retention
Related/similar drugs
ALERT: U.S. Boxed Warning
Suicidality and antidepressant drugs:Antidepressants increased the risk, compared with placebo, of suicidal thinking and behavior (suicidality) in short-term studies in children, adolescents, and young adults with major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of isocarboxazid or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared with placebo in adults older than 24 years; there was a reduction in risk with antidepressants compared with placebo in adults 65 years and older. Depression and certain other psychiatric disorders are associated with increases in the risk of suicide. Closely monitor and observe patients of all ages who are started on antidepressant therapy for clinical worsening, suicidality, or unusual changes in behavior. Advise families and caregivers of the need for close observation and communication with their health care provider. Isocarboxazid is not approved for use in pediatric patients.
Pooled analyses of short-term (4- to 16-weeks), placebo-controlled trials of 9 antidepressant drugs (selective serotonin reuptake inhibitors [SSRIs] and others) in children and adolescents with MDD, obsessive-compulsive disorder (OCD), or other psychiatric disorders (a total of 24 trials involving more than 4,400 patients) have revealed a greater risk of adverse reactions representing suicidal thinking or behavior (suicidality) during the first few months of treatment in those receiving antidepressants. The average risk of such reactions in patients receiving antidepressants was 4%, twice the placebo risk of 2%. No suicides occurred in these trials.
Warnings/Precautions
Major psychiatric warnings:
• Suicidal thinking/behavior: [US Boxed Warning]: Antidepressants increase the risk of suicidal thinking and behavior in children, adolescents, and young adults (18 to 24 years of age) with major depressive disorder (MDD) and other psychiatric disorders; consider risk prior to prescribing. Short-term studies did not show an increased risk in patients >24 years of age and showed a decreased risk in patients ≥65 years. Closely monitor patients for clinical worsening, suicidality, or unusual changes in behavior, particularly during the initial 1 to 2 months of therapy or during periods of dosage adjustments (increases or decreases); the patient’s family or caregiver should be instructed to closely observe the patient and communicate condition with health care provider. A medication guide concerning the use of antidepressants should be dispensed with each prescription. Isocarboxazid is FDA approved for the treatment of depression in children ≥16 years of age.
- The possibility of a suicide attempt is inherent in major depression and may persist until remission occurs. Patients treated with antidepressants should be observed for clinical worsening and suicidality, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases. Worsening depression and severe abrupt suicidality that are not part of the presenting symptoms may require discontinuation or modification of drug therapy. Use caution in high-risk patients during initiation of therapy.
- Prescriptions should be written for the smallest quantity consistent with good patient care. The patient's family or caregiver should be alerted to monitor patients for the emergence of suicidality and associated behaviors such as anxiety, agitation, panic attacks, insomnia, irritability, hostility, impulsivity, akathisia, hypomania, and mania; patients should be instructed to notify their healthcare provider if any of these symptoms or worsening depression or psychosis occur.
Concerns related to adverse effects:
• Hypertensive crisis: Cases of hypertensive crisis (sometimes fatal) have occurred; symptoms include severe headache, nausea/vomiting, neck stiffness/soreness, photophobia, and sweating. Monitor blood pressure closely in all patients. May occur with foods/supplements high in tyramine, tryptophan, phenylalanine, or tyrosine content; treatment with phentolamine is recommended for hypertensive crisis.
• Orthostatic hypotension: May cause orthostatic hypotension; use with caution in patients at risk of this effect or in those who would not tolerate transient hypotensive episodes (cerebrovascular disease, cardiovascular disease, hypovolemia, or concurrent medication use which may predispose to hypotension/bradycardia).
Disease-related concerns:
• Angina: MAO inhibitors may mask anginal pain.
• Diabetes: Use with caution in patients with diabetes mellitus; sensitization to the effects of insulin may occur, monitor blood glucose closely.
• Drug abuse: Use with caution in patients with a history of drug abuse or acute alcoholism; potential for drug dependency exists, especially in patients using excessive doses.
• Hepatic impairment: Use is contraindicated in patients with a history of liver disease or abnormal LFTs. Decreased hepatic function may result in decreased clearance and increased half-life and plasma concentrations. Consider switching to a different antidepressant class due to side effect profile, risk of worsening hepatotoxicity and apparent pharmacokinetic changes in chronic liver disease (Mullish 2014).
• Mania/hypomania: May precipitate a shift to mania or hypomania in patients with bipolar disorder. Monotherapy in patients with bipolar disorder should be avoided. Combination therapy with an antidepressant and a mood stabilizer may be effective for acute treatment of bipolar major depressive episodes, but should be avoided in acute mania or mixed episodes, as well as maintenance treatment in bipolar disorder due to the mood-destabilizing effects of antidepressants (CANMAT [Yatham 2018]; WFSBP [Grunze 2018]). Patients presenting with depressive symptoms should be screened for bipolar disorder. Isocarboxazid is not FDA approved for the treatment of bipolar depression.
• Renal impairment: Use with caution in patients with renal impairment; contraindicated in patients with severe impairment.
• Seizure disorder: Use with caution in patients at risk of seizures, including those with a history of seizures, head trauma, brain damage, alcoholism, or concurrent therapy with medications which may lower seizure threshold.
• Thyroid dysfunction: Use with caution in patients with hyperthyroidism.
Concurrent drug therapy issues:
• High potential for interactions: Do not use with other MAO inhibitors or antidepressants. Do not use within 5 weeks of fluoxetine discontinuation or 1 week of other antidepressant discontinuation. Avoid products containing sympathomimetic stimulants, dextromethorphan, disulfiram, and meperidine. Concurrent use with antihypertensive agents may lead to exaggeration of hypotensive effects.
• Sedatives: CNS effects may be potentiated when used with other sedative drugs or ethanol.
Special populations:
• Elderly: The MAO inhibitors are effective and generally well tolerated by older patients. It is the potential interactions with tyramine-containing foods and other drugs, and their effects on blood pressure that have limited their use.
• Hyperactive or agitated patients: Use with caution in patients who are hyperactive and/or hyperexcitable.
• Surgical patients: According to the manufacturer isocarboxazid use within 10 days prior elective surgery is contraindicated. The decision to continue or withhold MAO inhibitors must be done in collaboration with the patient's psychiatrist. Currently, an MAO-safe anesthetic technique which excludes the use of meperidine and indirect-acting adrenergic agonists is recommended for patients requiring continued MAO inhibitor therapy (Huyse 2006).
Other warnings/precautions:
• Appropriate use: Isocarboxazid is not generally considered a first-line agent for the treatment of depression; it is typically used in patients who have failed to respond to other treatments.
• Discontinuation syndrome: Abrupt discontinuation or interruption of antidepressant therapy has been associated with a discontinuation syndrome. Symptoms arising may vary with antidepressant however commonly include nausea, vomiting, diarrhea, headaches, lightheadedness, dizziness, diminished appetite, sweating, chills, tremors, paresthesias, fatigue, somnolence, and sleep disturbances (eg, vivid dreams, insomnia). Less common symptoms include electric shock-like sensations, cardiac arrhythmias (more common with tricyclic antidepressants), myalgias, parkinsonism, arthralgias, and balance difficulties. Psychological symptoms may also emerge such as agitation, anxiety, akathisia, panic attacks, irritability, aggressiveness, worsening of mood, dysphoria, mood lability, hyperactivity, mania/hypomania, depersonalization, decreased concentration, slowed thinking, confusion, and memory or concentration difficulties. Greater risks for developing a discontinuation syndrome have been associated with antidepressants with shorter half-lives, longer durations of treatment, and abrupt discontinuation. More severe symptoms have also been associated with MAO inhibitors. For antidepressants of short or intermediate half-lives, symptoms may emerge within 2 to 5 days after treatment discontinuation and last 7 to 14 days (APA 2010; Fav, 2006; Haddad 2001; Shelton 2001; Warner 2006).
• Electroconvulsive therapy: May increase the risks associated with electroconvulsive therapy; consider discontinuing, when possible, prior to ECT treatment.
• Myelography: Discontinue at least 48 hours prior to myelography.
Monitoring Parameters
Renal function (baseline, periodic); liver function (baseline, periodic); blood pressure, heart rate; mood, suicidal ideation (especially at the beginning of therapy or when doses are increased or decreased)
Pregnancy Considerations
Animal reproduction studies have not been conducted.
Pregnant women exposed to antidepressants during pregnancy are encouraged to enroll in the National Pregnancy Registry for Antidepressants (NPRAD). Women 18 to 45 years of age or their health care providers may contact the registry by calling 844-405-6185. Enrollment should be done as early in pregnancy as possible.
Patient Education
What is this drug used for?
• It is used to treat low mood (depression). It is most often given after other drugs have failed to help.
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
• Dry mouth
• Fatigue
• Constipation
• Trouble sleeping
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
• Depression like thoughts of suicide, anxiety, emotional instability, or confusion.
• Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin
• Weakness on 1 side of the body, trouble speaking or thinking, change in balance, drooping on one side of the face, or blurred eyesight
• Irritability
• Panic attacks
• Mood changes
• Agitation
• Chest pain
• Fast heartbeat
• Slow heartbeat
• Passing out
• Severe nausea
• Vomiting
• Headache
• Abnormal heartbeat
• Neck rigidity
• Sweating a lot
• Enlarged pupils
• Sensitivity to light
• Seizures
• Severe dizziness
• Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a limited summary of general information about the medicine's uses from the patient education leaflet and is not intended to be comprehensive. This limited summary does NOT include all information available about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not intended to provide medical advice, diagnosis or treatment and does not replace information you receive from the healthcare provider. For a more detailed summary of information about the risks and benefits of using this medicine, please speak with your healthcare provider and review the entire patient education leaflet.
Frequently asked questions
More about isocarboxazid
- Check interactions
- Compare alternatives
- Reviews (9)
- Side effects
- Dosage information
- During pregnancy
- Drug class: monoamine oxidase inhibitors
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.