RX 763 Pill - pink oval, 21mm
Pill with imprint RX 763 is Pink, Oval and has been identified as Amoxicillin 875 mg. It is supplied by Ranbaxy Pharmaceuticals Inc.
Amoxicillin is used in the treatment of Bacterial Infection; Bacterial Endocarditis Prevention; Actinomycosis; Anthrax Prophylaxis; Bladder Infection and belongs to the drug class aminopenicillins. There is no proven risk in humans during pregnancy. Amoxicillin 875 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for RX 763
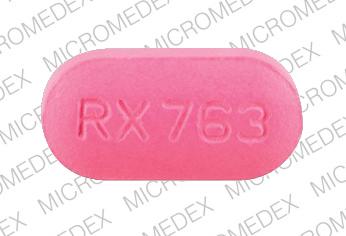

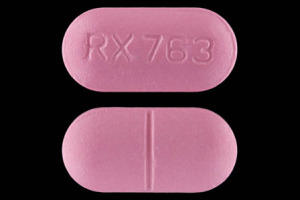

Amoxicillin
- Imprint
- RX 763
- Strength
- 875 mg
- Color
- Pink
- Size
- 21.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Aminopenicillins
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Ranbaxy Pharmaceuticals Inc.
- National Drug Code (NDC)
- 63304-0763 (Discontinued)
See also:
Cefdinir
Cefdinir is used for bacterial infection, bronchitis, middle ear infections, pneumonia, sinusitis ...
Amoxicillin/clavulanate
Amoxicillin and clavulanate potassium is a combination antibiotic used to treat bacterial ...
Botox
Botox is used cosmetically to reduce facial lines and wrinkles and for medical purposes for ...
Levofloxacin
Levofloxacin is a fluoroquinolone antibiotic used to treat serious bacterial infections and prevent ...
Augmentin
Augmentin is a prescription antibiotic combining amoxicillin and clavulanate to treat bacterial ...
Ceftriaxone
Ceftriaxone is used for bacteremia, bacterial endocarditis prevention, bacterial infection, bone ...
Clindamycin
Clindamycin (Cleocin) is used to treat serious infections caused by bacteria. Includes clindamycin ...
Metronidazole
Metronidazole is an antibiotic used to fight bacteria in your body. Learn about side effects ...
Ciprofloxacin
Ciprofloxacin is an antibiotic belong to a group of drugs called fluoroquinolones. Learn about side ...
Cephalexin
Cephalexin is a cephalosporin antibiotic used to treat bacterial infections like respiratory, skin ...
More about amoxicillin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (367)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: aminopenicillins
- Breastfeeding
- En español
Patient resources
Other brands
Amoxil, Moxatag, Trimox, Moxilin, ... +2 more
Professional resources
- Amoxicillin monograph
- Amoxicillin (FDA)
- Amoxicillin Capsules (FDA)
- Amoxicillin Chewable (FDA)
- Amoxicillin Extended Release Tablets (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
