MYLAN 6727 MYLAN 6727 Pill - purple blue capsule/oblong, 18mm
Pill with imprint MYLAN 6727 MYLAN 6727 is Purple / Blue, Capsule/Oblong and has been identified as Zonisamide 100 mg. It is supplied by Mylan Pharmaceuticals Inc.
Zonisamide is used in the treatment of Seizures; Epilepsy and belongs to the drug class carbonic anhydrase inhibitor anticonvulsants. Risk cannot be ruled out during pregnancy. Zonisamide 100 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for MYLAN 6727 MYLAN 6727
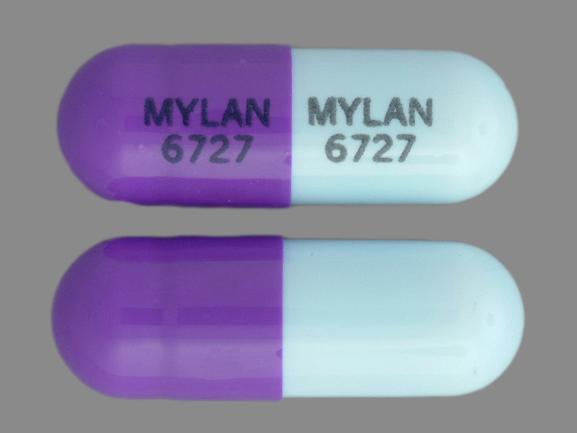
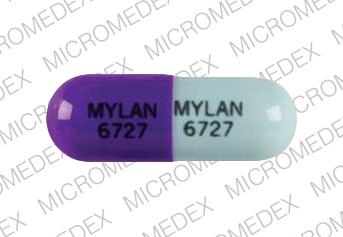
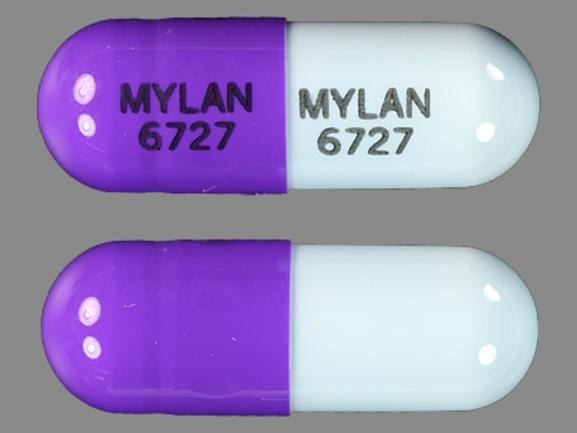
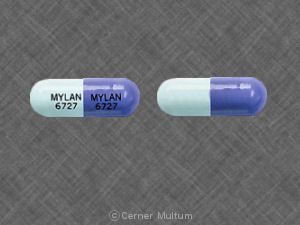
Zonisamide
- Imprint
- MYLAN 6727 MYLAN 6727
- Strength
- 100 mg
- Color
- Purple / Blue
- Size
- 18.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Carbonic anhydrase inhibitor anticonvulsants
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Mylan Pharmaceuticals Inc.
- Inactive Ingredients
-
lactose anhydrous,
ferrosoferric oxide,
silicon dioxide,
D&C Yellow No. 10,
D&C Red No. 28,
FD&C Blue No. 1,
FD&C Blue No. 2,
FD&C Red No. 40,
microcrystalline cellulose,
propylene glycol,
shellac,
sodium lauryl sulfate,
titanium dioxide,
FD&C Red No. 3,
gelatin,
magnesium stearate
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00378-6727 (Discontinued) | Mylan Pharmaceuticals Inc. |
| 51079-0768 (Discontinued) | UDL Laboratories Inc. |
See also:
Lyrica
Lyrica is used to control seizures, treat nerve pain and fibromyalgia. Learn about side effects ...
Lamictal
Lamictal is an anti-epileptic medication used treat seizures in adults and children over 2 years ...
Botox
Botox is used cosmetically to reduce facial lines and wrinkles and for medical purposes for ...
Depakote
Depakote is used to treat various types of seizure disorders. Learn about side effects ...
Carbamazepine
Carbamazepine is used to treat epileptic seizures and nerve pain such as trigeminal neuralgia ...
Diazepam
Diazepam is used to treat anxiety disorders, alcohol withdrawal symptoms, or muscle spasms. Learn ...
Levetiracetam
Levetiracetam is used for bipolar disorder, epilepsy, hyperekplexia, neuralgia, new daily ...
Topiramate
Learn about topiramate, an anticonvulsant used for seizures, migraine prevention, and weight loss ...
Pregabalin
Pregabalin may be used to treat certain types of pain and used in combination with other ...
Lamotrigine
Lamotrigine is used for anxiety, bipolar disorder, borderline personality disorder, cyclothymic ...
More about zonisamide
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (146)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: carbonic anhydrase inhibitor anticonvulsants
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
