barr 555 585 Pill - green round, 11mm
Pill with imprint barr 555 585 is Green, Round and has been identified as Chlorzoxazone 500 mg. It is supplied by Barr Laboratories, Inc.
Chlorzoxazone is used in the treatment of Muscle Spasm and belongs to the drug class skeletal muscle relaxants. FDA has not classified the drug for risk during pregnancy. Chlorzoxazone 500 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for barr 555 585


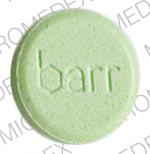

Chlorzoxazone
- Imprint
- barr 555 585
- Strength
- 500 mg
- Color
- Green
- Size
- 11.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Skeletal muscle relaxants
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Barr Laboratories, Inc.
- National Drug Code (NDC)
- 00555-0585 (Discontinued)
See also:
Flexeril
Flexeril (cyclobenzaprine) is a muscle relaxant used to treat skeletal muscle conditions such as ...
Valium
Valium is used to treat anxiety disorders, alcohol withdrawal symptoms, or muscle spasms. Learn ...
Dupixent
Dupixent is used to treat eczema, eosinophilic or oral-corticosteroid-dependent asthma, chronic ...
Robaxin
Robaxin (methocarbamol) is used to treat skeletal muscle conditions such as pain or injury ...
Zanaflex
Zanaflex (tizanidine) is a short-acting muscle relaxer used to treat spasticity by temporarily ...
Soma
Soma (carisoprodol) is used treat injuries and other painful muscle conditions. Includes Soma side ...
Diazepam
Diazepam is used to treat anxiety disorders, alcohol withdrawal symptoms, or muscle spasms. Learn ...
Baclofen
Baclofen is a muscle relaxant used to treat spasticity and muscle pain in conditions like multiple ...
Tizanidine
Tizanidine is a short-acting muscle relaxant and is used to treat spasticity. Learn about side ...
Methocarbamol
Methocarbamol is a muscle relaxant that works by blocking pain sensations. Includes methocarbamol ...
More about chlorzoxazone
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (62)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: skeletal muscle relaxants
- En español
Patient resources
Other brands
Paraflex, Parafon Forte DSC, Lorzone, Remular-S
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
