Revalor-G (Canada)
This treatment applies to the following species:Trenbolone acetate 40 mg /estradiol 8 mg
Veterinary Use Only
FOR PASTURE STEERS/HEIFERS (195-320 kg) AND FEEDLOT STEERS (215-330 kg)
DIN: 02246207
Description
Revalor®-G is an implant containing 40 mg of trenbolone acetate and 8 mg estradiol. Each implant consists of 2 small yellow pellets.
Each pellet contains:
Active ingredients: 20 mg trenbolone acetate, 4 mg estradiol.
Non-medicinal ingredients: 6.25 mg cholesterol, 0.5 mg magnesium stearate, 1.25 mg ethyl cellulose N200.
Therapeutic Classification
Growth promotant.
Revalor-G Indications
This product contains trenbolone acetate and estradiol in a slow release delivery system and is indicated to increase rate of weight gain in pasture steers/heifers weighing 195-320 kg and feedlot steers weighing 215-330 kg.
Revalor-G Dosage And Administration
Dosage form:
One implant containing 40 mg trenbolone acetate and 8 mg estradiol is administered to each animal. The 2 pellets which make up the dosage of Revalor®-G are contained in one division of the multiple dose cartridge. Ten doses are in each cartridge. The cartridge is designed to be used with the Revalor® implant gun.
Administration:
A) Site Of Administration
The implant is placed under the skin on the posterior aspect of the ear by means of the Revalor® implanter available from Intervet Canada Corp. After properly restraining the animal to allow access to the ear, cleanse the skin at the implant needle puncture site. Improperly cleansed skin may result in abscess formation and the subsequent loss of the implant. The implant is placed subcutaneously between the skin and the cartilage on the back, middle third of the ear below the midline (see Figure 1). The implant must not be placed closer to the head than the outer edge of the cartilage ring. The location of needle insertion is at a point corresponding to the center of the ear with the needle directed towards the head, and at least a needle length away from the intended deposition site (see Figure 2). Care should be taken to avoid injuring the major blood vessels or cartilage of the ear.
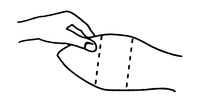
Figure 1
Ear of bovine ready for implantation.
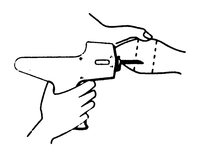
Figure 2
Rear view of the bovine ear showing the site for insertion of the implanter needle.
b) method of administration
1. Do not remove the cap (C) of the cartridge containing the implants.
2. Place the cartridge (D) (with the capped end to the front) into slot at the top of the implanter magazine (labelled A on the diagram).
3. Gently push the cartridge into the slot until it clicks into place.
4. The implanter is now ready for use.
5. Take the ear of the animal firmly with the free hand (in the manner shown in Figure 1) and insert the needle into the subcutaneous tissue at the point indicated (Figure 2). Press the trigger (E on Figure 3).
6. The retraction bar will automatically withdraw the needle from the ear aiding in depositing the pellets of the implant in a single row.
7. The gun automatically advances the cartridge to the next implant.
8. When all the implants have been administered, the cartridge will fall out of the bottom of the magazine and may be replaced by a new one.
9. To change the needle, loosen the needle locking nut (labelled F in Figure 3) and replace the needle. Tighten the nut finger tight and the implanter is ready for use.
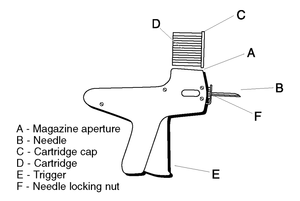
Figure 3
Diagram of the implanter and cartridge.
Revalor-G Cautions
Not for use in animals intended for subsequent breeding.
Warnings
Do not use in calves to be processed for veal. Implant pellets in the ear only. Do not attempt salvage of implant site for human or animal food. Do not use in dairy cattle. Keep out of reach of children.
Storage
Store the unopened product below 25 °C. Do not freeze. Store the opened product under refrigeration (2-8 °C). Do not use the opened product after 6 months.
HOW SUPPLIED
Box of 10 cartridges x 10 implants (100 implants).
Intervet Canada Corp., 16750, route Transcanadienne, Kirkland, QC H9H 4M7
1 888 306-0069
Intervet Canada Corp. is a subsidiary of Merck & Co., Inc
® Registered trademark of Intervet International B.V. Used under license.
September 9, 2011
CPN: 12081794
Intervet Canada Corp.
16750 ROUTE TRANSCANADIENNE, KIRKLAND, QC, H9H 4M7
| Order Desk: | 514-428-7013 | |
| Toll-Free: | 866-683-7838 | |
| Fax: | Toll-free 888-498-4444; local 514-428-7014 | |
| Website: | www.merck-animal-health.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
