Pronefra Palatable Oral Suspension (Canada)
This treatment applies to the following species:FOR CATS AND DOGS
VETERINARY HEALTH PRODUCT
SUPPORTS NORMAL FUNCTION AND HEALTH OF THE KIDNEYS
NN.O9F6
Description
PRONEFRA® Palatable Oral Suspension is recommended for cats and dogs for the support of normal renal health. PRONEFRA Oral Suspension has 4 main benefits:
● Calcium carbonate and magnesium carbonate help support normal renal function by maintaining healthy phosphorus levels.
● The fish protein hydrolysate may support normal blood pressure.
● Chitosan supports normal kidney function.
● Highly palatable liquid supplement, for an easy administration.
The formulation also contains: olive, colloidal silicon dioxide, chicken liver hydrolysate, coconut oil, polysorbate 80, d,dl-alpha tocopheryl acetate.
CAUTIONS: Do not exceed the recommended dose.
Recommend to seek veterinary opinion before an extended period of use.
DIRECTIONS FOR USE: SHAKE WELL BEFORE USE. Light sedimentation is normal.
For CATS: Administer orally 1 mL / 4 kg body weight twice daily
For DOGS: Administer orally 1 mL / 5 kg body weight twice daily
Give at mealtime or within 2 hours after eating. PRONEFRA Oral Suspension is highly palatable, allowing for easy administration.
May be mixed with food or given directly in the mouth.
|
Recommended dose according to cat body weight. Give dose twice a day. |
|
|
kg |
mL |
|
2 |
0.5 |
|
3 |
0.75 |
|
4 |
1 |
|
5 |
1.25 |
|
6 |
1.5 |
|
7 |
1.75 |
|
8 |
2 |
|
Recommended dose according to dog body weight. Give dose twice a day. |
|
|
kg |
mL |
|
2.5 |
0.5 |
|
5 |
1 |
|
10 |
2 |
|
15 |
3 |
|
20 |
4 |
|
25 |
5 |
Ensure drinking water is available at all times.
NOTE: The dosing syringe provided delivers 0.25 mL for every 1 kg increment (CATS) and 0.5 mL for every 2.5 kg increment (DOGS).
Storage
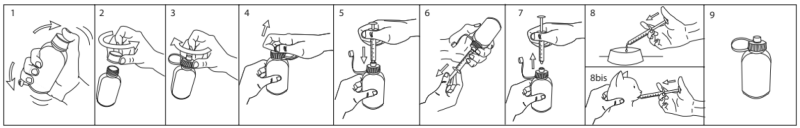
Always store product in a dry place at room temperature, even after opening. The product might freeze at low temperatures, but revert back to the original consistency after some hours at room temperature without impacting the quality of the product.ADVICE FOR CORRECT USE OF ADAPTER CAP (see illustrations below):
1: Shake well before use.
2: Unscrew the white cap to open the bottle.
3: Screw the adapter cap on the bottle.
4: Keep the bottle upright and open the adapter cap.
5: Insert the oral dosing syringe firmly inside.
6: Invert the bottle and slowly pull the plunger down so that the oral dosing syringe fills with the product. Withdraw the recommended quantity according to the weight of your animal. Pull the plunger down until the black line (corresponding to your pet’s body weight) is aligned with the bottom end of the syringe.
7: Return the bottle upright and remove the oral dosing syringe by gently twisting it out of the adapter cap. Close the adapter cap.
8: Insert the syringe into the mouth of your animal and gently push the product out of the syringe. Make sure that your animal swallows the product.
9: Always keep the bottle closed with the adapter cap. Thoroughly rinse the syringe after use with clear water. The product does not require refrigeration.
ADDITIONAL INFORMATION:
PRONEFRA Oral Suspension is available in two presentations: • 60 mL • 180 mL.
Warnings: Keep out of the reach of children and animals.
QUESTIONS? 1-800-338-3659 - ca.virbac.com
Made in France.
Imported and distributed in Canada by: Virbac Canada Inc., 209-231 Shearson Crescent, Cambridge, Ontario N1T 1J5
© 2021 Virbac Corporation. All Rights Reserved. PRONEFRA is a registered trademark of Virbac S.A.
12615, 12616
84143801
|
60 mL |
12615 |
84143701 84143601 |
|
180 mL |
12616 |
84144001 84143901 |
CPN: 1177073.3
209-231 SHEARSON CRESCENT, CAMBRIDGE, ON, N1T 1J5
| Toll-Free: | 866-458-3350 | |
| Fax: | 844-458-4004 | |
| Website: | https://ca.virbac.com/ |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
