Lutalyse Sterile Solution (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
Dinoprost Tromethamine Injection Mfr Std
Sterile solution for injection
Veterinary Use Only
DIN 00813605
DESCRIPTION: Lutalyse® Sterile Solution is a sterile injectable solution containing the naturally occurring prostaglandin F2α (PGF2α or dinoprost) as the tromethamine salt.
Each mL contains 5 mg dinoprost (as dinoprost tromethamine) and 16.5 mg benzyl alcohol as the preservative.
Lutalyse Sterile Solution Indications
Horses: Lutalyse Sterile Solution is indicated for its luteolytic effect on corpora lutea in mares to “induce” estrus and may be utilized to stimulate regression of the corpus luteum followed by return to estrus and/or ovulation in mares demonstrating extended diestrus.
Cattle: Lutalyse Sterile Solution is indicated for its luteolytic effect in cattle. This luteolytic action may be utilized to:
(1) Effectively control the time of estrus in cycling cattle that have a corpus luteum
(2) Treat sub-estrus (no visible estrus)
(3) Induce abortion in cattle from 5 to 130 days of gestation
(4) Treat chronic metritis and pyometra
(5) Induce parturition on or after day 270 of gestation
(6) Use with FACTREL® (gonadorelin hydrochloride) sterile solution to synchronize estrous cycles to allow fixed-time artificial insemination (FTAI) in lactating dairy cows
Swine: Lutalyse Sterile Solution may be used to induce parturition in swine when administered within 3 days (72 hours) of normal predicted farrowing dates.
Dosage and Administration
Horses: To induce estrus, administer 5 mg (1 mL) subcutaneously.
An examination of the genital tract should be conducted before and after administration of Lutalyse Sterile Solution. It should be realized that not all heats are fertile, and that ovulations can accompany silent estrus. Due to the seasonal polyestrus nature of the mare, the efficacy of Lutalyse Sterile Solution may vary with the time of year it is administered. In extended diestrus, there is failure to exhibit regular estrus cycles which should not be confused with true anestrus. Many mares described as anestrual during the breeding season have serum progesterone levels consistent with the presence of a functional corpus luteum. A proportion of “barren”, maiden, and lactating mares do not exhibit regular estrus cycles and may be in extended diestrus. Following abortion, early fetal death and resorption, or as a result of “pseudopregnancy”, there may be serum progesterone levels consistent with a functional corpus luteum.
Treatment of such mares with Lutalyse Sterile Solution usually results in regression of the corpus luteum followed by return of estrus and/or ovulation.
Cattle: To control estrus, to treat subestrus, pyometra, and mummified fetus and to induce abortion or parturition administer 25 mg (5 mL) intramuscularly.
(1) Effectively control the time of estrus in cycling cattle that have a corpus luteum: Cows and heifers treated during this time will return to estrus and ovulate within 2 or 4 days after treatment. (Note: administration of Lutalyse Sterile Solution to cattle within 5 days after estrus or 4 days before the onset of the next estrus may not influence the timing of the next estrus.) Rectal palpations and good records of estrus cycles are helpful for the most efficient use of Lutalyse Sterile Solution.
(2) Treat sub-estrus (no visible estrus): Individual cattle may have normal cyclical ovarian activity without detectable behavioral estrus; this occurs most frequently in the winter months, at peak lactation in high producing dairy cows, in cows receiving marginal nutrition and in suckled beef cows. If a corpus luteum is present and ovulation has not occurred in the previous 4 days, administration of Lutalyse Sterile Solution may result in corpus luteum regression followed by return to estrus and ovulation. Breeding of cattle treated with PGF2α for the above indication may be by natural service, artificial insemination at the usual time in relation to observed estrus, or by fixed time insemination 78 hours (75-80 hours) post-treatment.
(3) Induce abortion in cattle from 5 to 130 days of gestation: Up to day 70, abortion usually occurs in less than 4 days; between days 70 and 130 in less than 7 days, but beyond day 130 less than 60 percent may abort within 3 weeks. Injected heifers should be kept under surveillance and given assistance if necessary.
(4) To treat chronic metritis and pyometra: In the cow, chronic metritis frequently occurs as a sequel to an acute or sub-acute endometritis in the first 2 or 3 weeks postpartum; typically, there is an intermittent purulent or muco-purulent discharge. Pyometra is characterized by the retention of purulent fluid within the uterus. Luteal regression through the administration of Lutalyse Sterile Solution is followed by estrus, during which the uterus is evacuated and the uterine environment is relatively unfavorable to the bacteria involved in the infection. Treatment may have to be repeated after 10-12 days where the condition is longstanding.
(5) To induce parturition on or after day 270 of gestation: The interval from administration to parturition is 1 to 8 days (average 50-60 hours). Induction of parturition in cattle is indicated where there is risk of oversize calves or where early parturition is desired. In addition, induction is indicated where it is desired to terminate pregnancy complicated by miscellaneous conditions, such as mummified or macerated fetuses, hydrops amnii, hydroallantois, etc. Such fetuses may need manual help to complete their passage through the genital tract.
For use with FACTREL (gonadorelin hydrochloride) sterile solution to synchronize estrous cycles to allow fixed-time artificial insemination (FTAI) in lactating dairy cows: Administer 2 mL FACTREL Injection (100 mcg gonadorelin) per cow as an intramuscular injection in a treatment regimen with the following framework:
● Administer the first dose of FACTREL Injection (2 mL) at Day 0.
● Administer LUTALYSE (25 mg dinoprost, as dinoprost tromethamine) Sterile Solution by intramuscular injection 6-8 days after the first dose of FACTREL Injection.
● Administer a second dose of FACTREL Injection (2 mL) 30 to 72 hours after the LUTALYSE injection.
● Perform FTAI 0 to 24 hours after the second dose of FACTREL Injection, or inseminate cows on detected estrus using standard herd practices.
Below are three examples of treatment regimens for FTAI that fit within the dosage regimen framework described immediately above:
|
|
Example 1 |
Example 2 |
Example 3 |
|
Day 0 (Monday) |
1st FACTREL |
1st FACTREL |
1st FACTREL |
|
Day 7 (the following Monday) |
LUTALYSE |
LUTALYSE |
LUTALYSE |
|
Day 9 (Wednesday) |
2nd FACTREL + FTAI at 48 hours after LUTALYSE |
2nd FACTREL 48 hours after LUTALYSE |
2nd FACTREL 56 hours after LUTALYSE |
|
Day 10 (Thursday) |
|
FTAI 24 hours after 2nd FACTREL |
FTAI 18 hours after 2nd FACTREL |
Porcine: To induce parturition administer 10 mg (2 mL) intramuscularly.
Lutalyse Sterile Solution may be used to induce parturition in swine when administered within 3 days (72 hours) of normal predicted farrowing dates. This can be advantageously employed to control the time of farrowing for sows and gilts in late gestation. On average the normal length of gestation for sows is 115 days; therefore, Lutalyse Sterile Solution normally should be administered 112 days or later following breeding. However, gestation periods can vary in length from herd to herd and among different breeds of swine; so the normal predicted farrowing date for the herd in question should be determined before Lutalyse Sterile Solution is administered.
When Lutalyse Sterile Solution is administered 2 to 3 days before normal farrowing dates, 80 percent of the sows injected can be expected to begin farrowing within 40 hours after injection. The mean interval from injection to parturition is approximately 26 hours. (In general, the closer the injection time is to the normal farrowing time, the higher will be the percentage response by sows.) Since piglets from treated sows are born earlier than would normally be the case, it may be necessary to provide assistance at farrowing, especially to any small, weak piglets.
It is recommended that Lutalyse Sterile Solution only be used in a swine facility where adequate records are available on (1) the average length of the gestation period for the animals in that facility, and (2) the breeding date and projected farrowing date for each animal. This information is essential to determine the appropriate time for product administration. Treatment earlier than 3 days prior to normal farrowing dates may produce weak piglets resulting in reduced survival.
As with any multidose vial practise aseptic techniques in withdrawing each dose. Adequately clean and disinfect the vial closure prior to entry with a sterile needle. Injections should be made using appropriate aseptic technique.
CONTRA-INDICATIONS: For subcutaneous use only in the equine and intramuscular use only in the bovine and porcine. Do not administer intravenously.
CAUTIONS:
(1) PGF2α may produce abortion in pregnant mares, cows and sows.
(2) Since studies have not been conducted in horses suffering from acute and chronic respiratory diseases, PGF2α should be used with caution in such cases.
(3) Due to seasonally polyestrus nature of mares the efficacy of this drug will vary with the time of year it is administered.
(4) In cattle, PGF2α is ineffective when administered prior to day 5 after ovulation or within 4 days before the on-set of the next estrus.
(5) Parturition induction in swine earlier than 72 hours prior to the normal farrowing date may result in piglet mortality.
Warnings
Not for human use.
Treated cattle and swine must not be slaughtered for use in food for at least 2 days after the latest treatment with this drug. Treated cattle must not be slaughtered for use in food for at least 7 days after sequential use with FACTREL.
No milk withholding time is required when used according to the label.
This drug is not to be administered to horses that are to be slaughtered for use in food.
This product should be handled carefully to avoid accidental self-injection or contact with the skin or mucous membranes of the user.
Prostaglandins of the F2α type may readily be absorbed through the skin and may cause bronchospasms and/or miscarriage.
Pregnant women, women in childbearing age, asthmatics and people with other respiratory tract diseases should exercise extreme caution when handling this product such as wearing waterproof gloves.
Accidental spillage on the skin should be washed off immediately with water.
In case of accidental self-injection, seek medical advice and show the package insert to the doctor. Should respiratory distress result from accidental inhalation or injection, the inhalation of a rapidly acting bronchodilator is indicated.
Keep out of reach of children
Adverse Reactions
Horses: The administration, by the subcutaneous route, of PGF2α to mares may be followed by adverse events commencing within 10 minutes; these may consist of sweating, increase in heart rate and some abdominal discomfort, but they pass within half to one hour, without treatment.
Cattle: A low incidence of clostridial and other infections at the injection site has been reported following prostaglandin administration. Treated animals should be closely observed post injection and appropriate antibiotic therapy initiated at the first sign(s) of infection. Very rarely anaphylactic reactions have occurred after administration of the product. Overdose: The most frequently observed adverse event is increased rectal temperature at a 5X or 10X overdose. However, rectal temperature changes have been transient in all cases observed and have not been detrimental to the animal. Limited salivation has been seen in some instances.
Swine: Adverse events consisting of increased body temperature, increased respiratory rate, increased salivation, stimulation of defecation and urination, flushing of the skin and restlessness (arching of back, pawing and rubbing and gnawing in crate) have been reported following the administration of PGF2α in pregnant sows and gilts. These events tend to parallel the signs exhibited by sows prior to normal parturition, only they appear to be condensed in time. These effects are usually seen within 15 minutes of injection and disappear within one hour.
Clinical Pharmacology
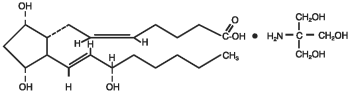
The empirical formula is C20H34O5•C4H11NO3.
The chemical structure of dinoprost tromethamine:
GENERAL BIOLOGIC ACTIONS: Prostaglandins occur in nearly all mammalian tissues. Prostaglandins, especially PGE’s, and PGF’s have been shown, in certain species, to (1) increase at time of parturition in amniotic fluid, maternal placenta, myometrium, and blood, (2) stimulate myometrial activity, and (3) to induce either abortion or parturition. Prostaglandins, especially PGF2α, have been shown to (1) normally increase in the uterus and blood to levels similar to that created by the administration of a dose of PGF2α, which was luteolytic, (2) be capable of crossing from the uterine vein to the ovarian artery (sheep), (3) be related to IUD induced luteal regression (sheep), and (4) be capable of regressing the corpus luteum of most mammalian species studied to date. Prostaglandins have been reported to result in release of pituitary tropic hormones. Data suggest prostaglandins, especially PGE’s and PGF’s, may be involved in the process of ovulation and gamete transport. Also PGF2α, has been reported to cause increase in blood pressure, bronchoconstriction, and smooth muscle stimulation in certain species.
SAFETY AND TOXICITY:
Horses: Overdose studies indicate that mares given 100 mg (20 times the recommended dose) daily for 8 days showed: (1) no change in heart rate, (2) an increase of 1.1°C in rectal temperature 5 hours after injection but had returned to normal by 15 to 24 hours after treatment, (3) no alteration in either 24 hour feed consumption or body weight, (4) decreases in digestive tract activity, sensitivity to pain, and general activity, with evidence of mouth and hind limb incoordination within one hour after injection, (5) laboured breathing and loose stool within one hour after injection, (6) profuse sweating for about 30 minutes; however, all characteristics, except rectal temperature, had returned to normal by 5 hours after injection.
Mares treated with a 160X overdose daily were recumbent within 10 to 30 minutes of injection but stood up of their own volition during the ensuing one to 4 hours. No mares died following administration of a 160X overdose of PGF2α daily for 8 days.
Cattle: In cattle, evaluation was made of clinical observations, clinical chemistry, hematology, urinalysis, organ weights, and gross plus microscopic measurements following treatment with various doses up to 250 mg PGF2α administered twice intramuscularly at a 10 day interval or doses of 25 mg administered daily for 10 days. There was no effect of PGF2α on the hematology or clinical chemistry parameters measured. A slight transitory increase in heart rate was detected. Rectal temperature was elevated about 0.8° C through the 6th hour after injection with 250 mg PGF2α but had returned to baseline at 24 hours after injection. Increased salivation occurred occasionally. No PGF2α associated gross lesions were detected at necropsy. There was no evidence of toxicological effects. At luteolytic doses, PGF2α did not impair fertility of cattle and had no effect on progeny. If given to a pregnant cow, it may cause abortion; the dose required for abortion varies considerably with the stage of gestation. The half life of PGF2α in bovine blood has been reported to be in the order of minutes. Assay of all muscle samples from treated animals were not significantly different from control tissue at 48 hours after injection in cattle.
Swine: PGF2α was administered intramuscularly to pregnant Yorkshire gilts between 111 and 113 days of gestation, at single doses of 10, 30, 50 and 100 mg of drug equivalent (4 gilts/group).
A control group, handled similarly, was injected with vehicle only. The safety of the drug to the pregnant gilts was assessed by the following parameters: clinical observations, food consumption, clinical pathologic determinations and 2 gilts/group were assessed for body weight changes, urinalysis, reproductive performance, organ weights and gross and microscopic observations. The results indicated no treatment related effects from PGF2α treatment that were deleterious to the health of the gilt or offspring.
PGF2α injected gilts had transient (from about 10 minutes to 3 hours) clinical signs that were consistent with previous literature reports which have been attributed to direct acting and/or central nervous system effects. The characteristic signs included: erythema, slight incoordination, nesting behaviour, itching, urination, abdominal muscle spasms, tail movements, hyperpnea, dyspnea, increased vocalization, salivation and at the 100 mg dose only, vomiting. PGF2α treatment did not have any effect on reproductive performance, gross and microscopic observations or the other parameters used to assess toxicosis. PGF2α is a synthetic natural prostaglandin. All systems associated with PGF2α metabolism exist in the body; therefore, no new metabolic, transport, excretory, binding or other systems need be established by the body to metabolize PGF2α.
Storage
Store between 15 and 25°C. Use contents within 90 days of first vial puncture. Particulate matter, usually identified as silica flakes may appear after periods of storage. These particles have altered neither the potency nor sterility of Lutalyse Sterile Solution.PRESENTATION: Lutalyse Sterile Solution is available in 30 mL and 100 mL glass vials.
Zoetis®, Lutalyse and Factrel are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
10026557-11-1
40041996
December 2023
CPN: 1198298.10
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
