GastroGard (Canada)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
(omeprazole USP)
37% w/w Oral Paste for the Treatment and Prevention of Equine Gastric Ulcers
DIN 02250349
FOR VETERINARY USE ONLY
Oral Paste for Horses and Foals
GastroGard Indications
● To aid in improving, healing and preventing the occurrence and recurrence of gastric ulcers, in horses and foals nine weeks of age and older.
Description
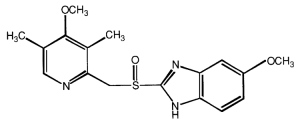
● Chemical name:
5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole.
Empirical formula: C17H19N3O3S. Molecular weight: 345.42. Structural formula:
NET CONTENTS:
● GastroGard® (omeprazole) Paste for horses contains 37% w/w omeprazole and is available in an adjustable-dose syringe. Each syringe contains 2.28 g of omeprazole. Syringes are calibrated according to bodyweight and are available in boxes of 7 and 72 units.
Storage
● Store below 25°C. Transient exposure to temperatures up to 30°C is permitted. Keep from freezing.
DOSAGE:
● For the treatment of gastric ulcers, GastroGard® Paste should be administered orally once-a-day for 28 days (4 weeks) at the recommended dosage of 4 mg omeprazole/kg body weight. To aid in the prevention of recurrence of gastric ulcers, continue treatment for an additional 28 days (4 weeks) by administering GastroGard® Paste at the recommended dose of 2 mg/kg. For the prevention of occurrence of gastric ulcers, GastroGard® Paste should be administered orally once-a-day at the recommended dosage of 1 mg omeprazole/kg body weight for up to 28 days or as prescribed by a veterinarian.
Directions For Use
● GastroGard® Paste for horses is recommended for use in horses and foals nine weeks of age and older. The contents of one syringe will dose a 575 kg horse at the rate of 4 mg omeprazole/kg body weight. For treatment of gastric ulcers, each weight marking on the syringe plunger will deliver sufficient omeprazole to treat 100 kg body weight. To aid in the prevention of recurrence of gastric ulcers, each weight marking will deliver sufficient omeprazole to dose 200 kg body weight. For the prevention of occurrence of gastric ulcers, each weight marking on the syringe plunger will deliver sufficient omeprazole to treat 400 kg body weight.
● To deliver GastroGard® Paste at the treatment dose rate of 4 mg omeprazole/kg body weight, set the syringe plunger to the appropriate weight marking according to the horse’s weight in kg.
● To deliver GastroGard® Paste at the dose rate of 2 mg omeprazole/kg body weight to aid in the prevention of recurrence of gastric ulcers, set the syringe plunger to the weight marking corresponding to half of the horse’s weight in kg.
● To deliver GastroGard® Paste at the dose rate of 1 mg omeprazole/kg body weight to aid in the prevention of occurrence of gastric ulcers, set the syringe plunger to the weight marking corresponding to a quarter of the horse’s weight in kg.
● To set the syringe plunger, unlock the knurled ring by rotating it 1/4 turn. Slide the knurled ring along the plunger shaft so that the arrow on the plunger indicating the prescribed weight marking is aligned with the notch on the ring. Rotate the knurled ring 1/4 turn to lock it in place and ensure it is locked. Remove the plastic cap from the tip of the nozzle before administering. Make sure the horse’s mouth contains no feed. Insert the syringe into the horse’s mouth at the interdental space. Depress the plunger until stopped by the knurled ring. The dose should be deposited on the back of the tongue or deep into the cheek pouch. Care should be taken to ensure that the horse consumes the complete dose. Treated animals should be observed briefly after administration to ensure that part of the dose is not lost or rejected. If any of the dose is lost, redosing is recommended.
● If, after dosing, the syringe is not completely empty, it may be re-used on following days until emptied. Replace the cap after each use.
Warnings
● This drug is not to be administered to horses that are to be slaughtered for use in food. Keep out of reach of children. In case of ingestion, contact a physician. Physicians may contact a poison control center for advice concerning accidental ingestion.
Adverse Reactions
● In efficacy trials, when the drug was administered at 1 mg omeprazole/kg for up to 30 days, or at 4 mg omeprazole/kg body weight daily for 28 days and 2 mg omeprazole/kg body weight daily for 30 additional days, no adverse reactions were observed.
Clinical Pharmacology
● Mechanism of action: Omeprazole is a gastric acid pump inhibitor that regulates the final step in hydrogen ion production and blocks gastric acid secretion regardless of the stimulus. Omeprazole irreversibly binds to the gastric parietal cell’s H+, K+ ATPase enzyme which pumps hydrogen ions into the lumen of the stomach in exchange for potassium ions. Since omeprazole accumulates in the cell cannaliculi and is irreversibly bound to the effect site, the plasma concentration at steady state is not directly related to the amount that is bound to the enzyme. The relationship between omeprazole action and plasma concentration is a function of the rate-limiting process of H+, K+ ATPase activity/turnover. Once all of the enzyme becomes bound, acid secretion resumes only after new H+, K+ ATPase is synthesized in the parietal cell (i.e., the rate of new enzyme synthesis exceeds the rate of inhibition).
● Pharmacodynamics: In a study of pharmacodynamic effects using horses with gastric cannulae, secretion of gastric acid was inhibited in horses given 4 mg omeprazole/kg/day. After the expected maximum suppression of gastric acid secretion was reached (5 days), the actual secretion of gastric acid was reduced by 99%, 95%, and 90% at 8, 16, and 24 hours, respectively.
● Pharmacokinetics: In a pharmacokinetic study involving thirteen healthy, mixed breed horses (8 female, 5 male) receiving multiple doses of omeprazole paste (4 mg/kg once daily for fifteen days) in either a fed or fasted state, there was no evidence of drug accumulation in the plasma when comparing the extent of systemic exposure (AUC0-∞). When comparing the individual bioavailability data (AUC0-∞ Cmax, and Tmax measurements) across the study days, there was great inter- and intrasubject variability in the rate and extent of product absorption. Also, the extent of omeprazole absorption in horses was reduced by approximately 67% in the presence of food. This is evidenced by the observation that the mean AUC0-∞ values measured during the fifth day of omeprazole therapy when the animals were fasted for 24 hours was approximately three times greater than the AUC estimated after the first and fifteenth doses when the horses were fed hay ad libitum and sweet feed (grain) twice daily. Prandial status did not affect the rate of drug elimination. The terminal half-life estimates (N=38) ranged from approximately one-half to eight hours.
EFFICACY:
● Dose Confirmation: GastroGard® Paste, administered to provide omeprazole at 4 mg/kg daily for 28 days, effectively healed or reduced the severity of gastric ulcers in 92% omeprazole-treated horses. In comparison, 32% of controls exhibited healed or less severe gastric ulcers. Horses enrolled in this study were healthy animals confirmed to have gastric ulcers by gastroscopy. Subsequent daily administration of GastroGard® Paste to provide omeprazole at 2 mg/kg for 30 days prevented recurrence of gastric ulcers in 84% of treated horses, whereas gastric ulcers recurred or became more severe in horses removed from omeprazole treatment. Additionally, GastroGard® Paste, when administered to provide omeprazole at 1 mg/kg daily to healthy horses confirmed not to have gastric ulcers by gastroscopy, effectively prevented the occurrence of gastric ulcers in 84% omeprazole-treated horses when submitted to ulcerogenic conditions for four weeks. In comparison, 76% of controls developed gastric ulcers.
● Clinical Field Trials: GastroGard® Paste administered at 4 mg/kg daily for 28 days healed or reduced severity of gastric ulcers in 99% of omeprazole-treated horses. In comparison, 32.4% of control horses had healed gastric ulcers or gastric ulcers which were reduced in severity. GastroGard® Paste administered at 1 mg/kg daily for 28 days, prevented the occurrence of gastric ulcers in 81% of omeprazole-treated horses. In comparison, 77% of control horses developed gastric ulcers. GastroGard® Paste administered at 1 mg/kg daily for 7 days during which the horses were submitted to ulcerogenic conditions, prevented the occurrence of gastric ulcers in 88% of omeprazole-treated horses. In comparison, 72% of control horses developed gastric ulcers during that period of time. These trials included horses of various breeds and under different management conditions, and included horses in race or show training, pleasure horses, and foals as young as one month. Horses enrolled in the various efficacy trials were healthy animals; a gastroscopy was performed on each one to assess the presence or absence of gastric ulcers before the onset of the trials. In these field trials, horses readily accepted GastroGard® Paste.
There were no drug related adverse reactions.
In the clinical trials, GastroGard® Paste was used concomitantly with other therapies, which included: anthelmintics, antibiotics, non-steroidal and steroidal anti-inflammatory agents, diuretics, tranquilizers and vaccines. In field trials involving 367 horses, including foals as young as one month of age, no adverse reactions attributable to omeprazole treatment were noted.
● Diagnostic and Management Considerations: The following clinical signs may be associated with gastric ulceration in adult horses: inappetence or decreased appetite, recurrent colic, intermittent loose stools or chronic diarrhea, poor hair coat, poor body condition, or poor performance. Clinical signs in foals may include: bruxism (grinding of teeth), excess salivation, colic, cranial abdominal tenderness, anorexia, diarrhea, sternal recumbency or weakness. A more accurate diagnosis of gastric ulceration in horses and foals may be made if gastric ulcers are visualized directly by gastroscopic examination of the gastric mucosa. Gastric ulcers may recur in horses if therapy to prevent recurrence is not administered after the initial treatment is completed. Use GastroGard® Paste at 2 mg omeprazole/kg body weight for the prevention of gastric ulcer recurrence following treatment, and at 1 mg omeprazole/kg body weight for the prevention of occurrence in horses with no recent history of gastric ulcers. The safety of administration of GastroGard® Paste for longer than 91 days has not been determined. Maximal acid suppression occurs after three to five days of treatment with omeprazole.
Consult your veterinarian for the diagnosis, treatment and control of gastric ulcers.
SAFETY:
● GastroGard® Paste was well tolerated in the following controlled efficacy and safety studies:
● In a placebo controlled adult horse safety study, horses received 20 mg/kg/day omeprazole (5x the recommended dose) for 90 days. No treatment related adverse effects were observed.
● In a placebo controlled tolerance study, adult horses were treated with GastroGard® Paste at a dosage of 40 mg/kg/day (10x the recommended dose) for 21 days. No treatment related adverse effects were observed.
● A placebo controlled foal safety study evaluated the safety of omeprazole at doses of 4, 12 or 20 mg/kg (1, 3, or 5x) once daily for 91 days. Foals ranged in age from 66 to 110 days at study initiation. Gamma glutamyltransferase (GGT) levels were significantly elevated in horses treated at exaggerated doses of 20 mg/kg (5x the recommended dose). Mean stomach to body weight ratio was higher for foals in the 3x and 5x groups than for controls; however, no abnormalities of the stomach were evident on histological examination.
REPRODUCTIVE SAFETY:
● In a male reproductive safety study, 10 stallions received GastroGard® Paste at 12 mg/kg/day (3x the recommended dose) for 70 days. No treatment related adverse effects on semen quality or breeding behavior were observed. A safety study in breeding mares has not been conducted.
CAUTIONS:
● The safety of GastroGard® Paste has not been determined in breeding, pregnant or lactating mares.
● Omeprazole is metabolized by the cytochrome P450 system, mainly in the liver. In humans, prolonged elimination of drugs metabolized through the cytochrome P450 system, including benzodiazepines, occurs with concomitant use of omeprazole. The clinical significance of these findings in horses have not been investigated. Horses with hepatic disease or a history of hepatic disease should be carefully monitored.
FOR MORE INFORMATION:
● Please call 1-888-637-4251
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
GastroGard® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
147613-004
CPN: 1182111.7
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
