Excede 100 Sterile Suspension (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
ceftiofur crystalline free acid sterile injectable suspension
Veterinary Use Only
DIN 02305453
Description
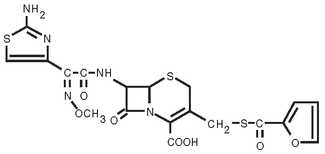
EXCEDE 100 Sterile Suspension is a ready-to-use formulation that contains the crystalline free acid of ceftiofur (CCFA), the designation for 7-[[2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]- 3 -[[(2-furanylcarbonyl)thio] methyl]-8-oxo-5-thia-1- azabicyclo[4.2.0]oct-2-ene 2-carboxylic acid.
Ceftiofur crystalline free acid is a broad spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria including β-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal, in vitro, resulting from inhibition of cell wall synthesis.
Each mL contains 100 mg ceftiofur equivalents (as ceftiofur crystalline free acid).
Figure 1. The chemical structure of ceftiofur crystalline free acid:
Excede 100 Sterile Suspension Indications
For intramuscular administration in the post-auricular region of the neck of swine (behind the bottom of the base of the ear). For the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis.
DOSAGE: Administer as a single intramuscular (IM) injection in the post-auricular region of the neck at a dose of 5 mg ceftiofur equivalents per kg body weight (1 mL per 20 kg body weight) with no more than 2 mL injected in a single injection site. Pigs heavier than 40 kg will require more than one injection.
Most animals will respond to treatment within three to five days. If no improvement is observed, the diagnosis should be reevaluated.
ADMINISTRATION: EXCEDE 100 Sterile Suspension is to be administered as a single intramuscular injection in the post-auricular region of the neck, with no more than 2 mL administered in a single injection site. Shake bottle vigorously for at least 30 seconds before using. If the bottle is left sitting for more than 5 minutes, shake again to resuspend the formulation.
Clinical Pharmacology
Swine: Ceftiofur administered as either ceftiofur sodium (EXCENEL® brand of ceftiofur sodium sterile powder), ceftiofur hydrochloride (EXCENEL® RTU brand of ceftiofur hydrochloride sterile suspension) or ceftiofur crystalline free acid (CCFA; EXCEDETM 100 Sterile Suspension) is metabolized rapidly to desfuroylceftiofur, the primary metabolite. Administration of ceftiofur to swine as CCFA at a single IM dosage of 5.0 mg ceftiofur-equivalents (CE)/kg body weight (BW) provides concentrations of ceftiofur and desfuroylceftiofur-related metabolites in plasma above the MIC90 for the swine respiratory disease (SRD) label pathogens Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis and Streptococcus suis for an extended period of time (see Figure 2 and Table 2).
Figure 2. Average plasma concentrations of ceftiofur- and desfuroylceftiofur-related metabolites for CCFA (EXCEDE 100 Sterile Suspension) after IM administration of 5.0 mg CE/kg BW and those for ceftiofur sodium (EXCENEL® brand of ceftiofur sodium sterile powder) after IM administration at 3 mg CE/kg BW for three consecutive days.
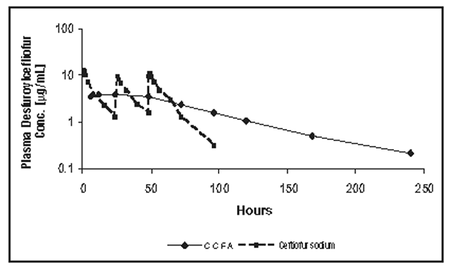
Table 1: Pharmacokinetic parameters in swine after a single IM administration of EXCEDE 100 Sterile Suspension at 5.0 mg ceftiofur equivalents (CE)/kg BW.
|
Pharmacokinetic Parameter |
Mean Value ± Standard Deviation |
|
Cmax (µg/mL) |
4.17 ± 0.92 |
|
tmax (h) |
22.0 ± 12.2 |
|
AUC 0-LOQ (µg•h/mL) |
373.0 ± 56.1 |
|
t1/2 (h) |
49.6 ± 11.8 |
Cmax = maximum plasma concentration (in µg CE/mL)
tmax = the time after injection when Cmax occurs (in hours)
AUC0-LOQ = the area under the plasma concentration vs. time curve from time of injection to the limit of quantitation of the assay (0.15 µg CE/mL)
t1/2 = terminal phase biological half life (in hours)
MICROBIOLOGY: Ceftiofur has demonstrated in vitro and in vivo activity against Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis and Streptococcus suis, the four major pathogenic bacteria associated with swine respiratory disease. In vitro activity has also been demonstrated against Salmonella choleraesuis, another bacterial pathogen associated with SRD; the clinical significance of this is not known.
A summary of minimum inhibitory concentrations (MIC) for various swine pathogens is presented in Table 2. Isolates were obtained in North America in the period 1992-2000. Testing followed Clinical and Laboratory Standards Institute (CLSI) Guidelines.1
Table 2. Ceftiofur MIC values from field studies evaluating SRD in the U.S. (1996/1997 and 2000/2001)
|
Pathogen |
Number of isolates |
MIC90* (µg/mL) |
MIC Range (µg/mL) |
|
A. pleuropneumoniae |
28 |
0.03 |
0.03-0.06 |
|
P. multocida |
58 |
0.03 |
0.03+ |
|
S. suis |
41 |
0.12 |
0.03-0.5 |
|
H. parasuis** |
72 |
0.06 |
0.03-0.25 |
* MIC for 90% of the isolates
** these MIC data were obtained using CLSI procedures but quality control values for H. parasuis had not been standardized
+ No range, all isolates yielded the same value
Table 3. Minimum Inhibitory Concentrations for Ceftiofur Against SRD Clinical Isolates (1992-2001)
|
Origin of strains |
Year |
Pathogens (number of strains) |
MIC range (µg/mL) |
MIC901(µg/mL) |
|
US |
1992-1993 |
A. pleuropneumoniae (50) |
≤ 0.03 |
≤ 0.03 |
|
P. multocida (50) |
≤ 0.03 - 0.06 |
≤ 0.03 |
||
|
S. suis (50) |
≤ 0.03 - 1.0 |
0.13 |
||
|
Canada |
1992-1993 |
A. pleuropneumoniae (10) |
≤ 0.03 |
≤ 0.03 |
|
S. suis (22) |
≤ 0.03 - 0.25 |
0.25 |
||
|
US |
1997-1998 |
H. parasuis (72) |
0.0039 - 0.25 |
0.06 |
|
US |
1997-1998 |
A. pleuropneumoniae (97) |
≤ 0.03 - ≤ 0.03 |
≤ 0.03 |
|
P. multocida (114) |
≤ 0.03 - 1.0 |
≤ 0.03 |
||
|
S. suis (106) |
≤ 0.03 - 4.0 |
0.5 |
||
|
US |
1998-1999 |
A. pleuropneumoniae (111) |
≤ 0.03 - 0.25 |
≤ 0.03 |
|
P. multocida (147) |
≤ 0.03 - 0.5 |
≤ 0.03 |
||
|
S. suis (142) |
≤ 0.03 - 1.0 |
0.25 |
||
|
US |
1999-2000 |
A. pleuropneumoniae (126) |
≤ 0.03 - 0.06 |
≤ 0.03 |
|
P. multocida (173) |
≤ 0.03 - 0.06 |
≤ 0.03 |
||
|
S. suis (146) |
≤ 0.03 - 4.0 |
0.06 |
||
|
S. choleraesuis (96) |
0.03 - > 4.0 |
1.0 |
||
|
US |
2000-2001 |
A. pleuropneumoniae (89) |
0.03 - 0.06 |
0.03 |
|
P. multocida (186) |
0.03 - 0.12 |
0.03 |
||
|
S. suis (167) |
0.03 - 4.0 |
0.06 |
1 Minimum inhibitory concentration for 90% of the isolates
Ceftiofur breakpoints have been established for bacterial SRD pathogens based on pharmacokinetic studies conducted with ceftiofur sodium and hydrochloride in swine after a single intramuscular injection of 3.0 to 5.0 mg CE/kg BW, and MIC and disk (30 µg) diffusion data. The following breakpoints are recommended by NCCLS.
|
Zone Diameter (mm) |
MIC (µg/mL) |
Interpretation |
|
≥21 |
≤2.0 |
(S) Susceptible |
|
18-20 |
4.0 |
(I) Intermediate |
|
≤17 |
≥8.0 |
(R) Resistant |
“Susceptible” or “S” indicates that the pathogen is likely to be inhibited by generally achievable blood concentrations after treatment with ceftiofur sodium, ceftiofur hydrochloride or ceftiofur crystalline free acid. “Intermediate” or “I” is a technical buffer zone and isolates falling into this category should be retested. Alternatively the organism may be successfully treated if the infection is in a body site where drug is physiologically concentrated. “Resistant” or “R” indicates that the achievable drug concentrations are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures1 require the use of laboratory control organisms for both standardized diffusion techniques and standardized dilution techniques. The 30 µg ceftiofur sodium disk should give the following zone diameters and the ceftiofur sodium standard reference powder (or disk) should provide the following MIC values for the reference strains. Ceftiofur sodium disks or powder reference standard are appropriate for both ceftiofur salts and CCFA.
|
QC Strain |
MIC (µg/mL) |
Disk Zone Diameter (mm) |
|
E. coli ATCC 25922 |
0.25-1.0 |
26-31 |
|
S. aureus ATCC 29213 |
0.25-1.0 |
- |
|
S. aureus ATCC 25923 |
- |
27-31 |
|
P. aeruginosa ATCC 27853 |
16.0-64.0 |
14-18 |
EFFICACY: The efficacy of EXCEDE 100 Sterile Suspension was evaluated in well-controlled clinical efficacy studies.
A challenge model study was conducted to evaluate the efficacy of EXCEDE 100 Sterile Suspension and select an appropriate dose for field testing. Pigs were challenged with an intratracheal administration of Actinobacillus pleuropneumoniae. EXCEDE 100 Sterile Suspension was administered as a single IM dose injected in the post-auricular region of the neck, 3 hours post-inoculation. Control pigs received a placebo injection. Mortality rates and lung lesion scores were statistically lower (25.0% and 28.3, p<0.05) for the EXCEDE 100 Sterile Suspension -treated groups compared with the placebo-treated control group (89.9% and 71.4). A dose range of 5.0 to 7.0 mg CE/kg BW was selected for further field testing.
The efficacy of a single dose of 5.0 or 7.0 mg CE/kg BW EXCEDE 100 Sterile Suspension for the treatment of SRD was confirmed in a well-controlled, multi-location field study. A total of 706 pigs with clinical signs of bacterial respiratory disease were enrolled and treated with a placebo injection or EXCEDE 100 Sterile Suspension administered as a single IM injection in the post-auricular region of the neck. Clinical observations were performed on Days 1-7 and rectal temperatures were taken on Days 1, 3, and 6 following treatment (Day 0). Necropsies were performed on all pigs that died during the study and after euthanasia of all remaining study pigs at the end of the 14-day post-enrollment study period. Lung lesions were scored and lungs were submitted for bacterial identification. Mortality rates were numerically lower (but not statistically different) for the EXCEDE 100 Sterile Suspension -treated groups (4.3% for the 5.0 mg CE/kg BW group and 4.2% for the 7.0 mg CE/kg BW group) compared with the placebo-treated control group (6.3%). There was a statistically significant (p<0.05) improvement in clinical cure rates for the EXCEDE 100 Sterile Suspension -treated groups (24.8% for the 5.0 mg CE/kg BW group and 26.4% for the 7.0 mg CE/kg BW group) compared with the placebo-treated control group (17.7%). Lung lesion scores were numerically higher (but not statistically different) for the EXCEDE 100 Sterile Suspension -treated groups (10.4% for both the 5.0 mg CE/kg BW and the 7.0 mg CE/kg BW group) compared with the placebo-treated control group (9.2%). Since the physical restraint of the study pigs was suspected to contribute to the increases in rectal temperatures observed, clinical cure rates were assessed after exclusion of the rectal temperature variable. With the revised clinical cure rate analysis, there was a statistically significant (p=0.02) improvement in clinical cure rates for the EXCEDE 100 Sterile Suspension -treated groups (42% for the 5.0 mg CE/kg BW group and 40% for the 7.0 mg CE/kg BW group) compared with the placebo-treated control group (30%). Bacteriological culture of the lungs of study pigs identified the following ceftiofur-susceptible respiratory pathogens: Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, and Streptococcus suis.
ANIMAL SAFETY: After parenteral administration, CCFA, ceftiofur sodium and ceftiofur hydrochloride all accede to the same principal metabolite, desfuroylceftiofur. Therefore, studies conducted with ceftiofur sodium are adequate to evaluate the systemic safety of CCFA. Results from a five-day tolerance study in normal feeder pigs indicated that ceftiofur sodium was well tolerated when administered at 125 mg/kg (more than 25 times the recommended dosage of CCFA) BW for five consecutive days. Ceftiofur sodium administered intramuscularly to pigs produced no overt adverse signs of toxicity. To determine the safety margin of ceftiofur in swine, a safety-toxicity study was conducted. Five barrows and five gilts per group were administered ceftiofur sodium intramuscularly at 0, 5, 15, 25 mg CE/kg BW for 15 days. This is 0, 1, 3 and 5 times the daily dose for CCFA and for 15 consecutive days compared with one day. There were no adverse systemic effects observed, indicating that ceftiofur has an adequate margin of safety when injected intramuscularly into feeder pigs.
A pivotal study evaluated the injection site tissue tolerance of EXCEDE 100 Sterile Suspension in swine when administered intramuscularly as a single injection at the maximum recommended dose volume of 2 mL (approximately 5 mg CE/kg BW) per injection site. EXCEDE 100 Sterile Suspension was injected intramuscularly into each side of the neck of sick swine at a dose volume of 2 mL/injection site. Clinical observations were made daily. At 3, 7 and 10 days post-injection, pairs of animals were euthanized and the neck injection sites were dissected for pathological examination (4 injection sites per time point). The injections were well tolerated in all pigs. Clinically, injection site reactions ranged from non detectable (6 of 12 sites) to a transitory (up to 4 days post-injection) palpable, non visible swelling (2 of 12 sites) or a small, visible, reddened nodule at the needle insertion point (4 of 12 sites; 3 of 4 nodules were barely detectable by 3 to 7 days post-injection). There was no clinical evidence of the injections at 10 days post-injection. At necropsy, half of the injection sites at both 3 and 7 days post-injection were scored as “negative” for irritation and the other half were scored as “slight irritation”. One animal had a visible lesion described as an area of tan with red speckles present in the deep muscle fascia, less than 6 cm2, at 10 days post-injection; this lesion and the remaining injection sites evaluated at 10 days post-injection were scored as “negative” for irritation.
A separate study evaluated the injection site tissue tolerance of EXCEDE 100 Sterile Suspension when administered intramuscularly into each side of the neck at volumes of 2.7 to 5.2 mL/injection site. The animals were killed and the neck injection sites were dissected at 21, 28, 42, and 56 days post-injection (4 injection sites/time point). Areas of discoloration with cystic cavities (≤ 1 mm diameter) containing clear, oily material were observed grossly in the fascia at necropsy. These were observed in 3/4 sites at 21 days post-injection; 2/4 sites at 28 days; and 3/4 sites at 42 days. Injection of volumes greater than 2 mL/injection site could extend the duration of possible local irritant effects, and could lead to carcass trim at the site of injection.
ADVERSE EFFECTS:
A pivotal injection site tolerance study demonstrated that EXCEDE 100 Sterile Suspension is well tolerated in pigs. Half of the injection sites at both 3 and 7 days post-injection were scored as “negative” for irritation and the other half were scored as “slight irritation”. All gross observations and measurements of injection sites qualified the sites at 10 days post-injection as “negative” for irritation. A separate study conducted with dose volumes of 2.7 to 5.2 mL per injection site demonstrated that EXCEDE 100 Sterile Suspension was well tolerated with swelling at the injection site noted in one animal. All injection sites examined grossly were scored as having no irritation.
No adverse effects were observed in multi-location field efficacy studies involving more than 1000 pigs.
Warning
• Treated swine must not be slaughtered for use in food for at least 49 days after the latest treatment with this drug.
• Non-observed withdrawal period, use of dosages in excess of those indicated or administration by unapproved routes may lead to illegal residues in tissues.
• Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth and clothing. Sensitization of the skin may be avoided by wearing latex gloves. Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention.
• To limit the development of antimicrobial resistance:
• EXCEDE 100 Sterile Suspension should not be used as a mass medication.
• EXCEDE 100 Sterile Suspension should only be used to treat individual cases of swine respiratory disease.
• The choice of EXCEDE 100 Sterile Suspension as the most appropriate treatment should be confirmed by clinical experience supported where possible, by pathogen culture and drug susceptibility testing.
• The extra-label use of Excede 100 Sterile Suspension is not recommended.
• KEEP OUT OF REACH OF CHILDREN.
The material safety data sheet contains more detailed occupational safety information. To report adverse effects in users, to obtain more information or to obtain a material safety data sheet, call 1-800-461-0917.
Contraindications
The use of EXCEDE 100 Sterile Suspension is contraindicated in animals previously found to be hypersensitive to the drug.
CAUTIONS: In the absence of studies on the safety of ceftiofur in pregnant swine or swine intended for breeding, such use is not recommended. EXCEDE 100 Sterile Suspension may induce a local tissue reaction at the injection site that may result in trim loss of edible tissue at slaughter. Do not inject more than 2 mL per injection site as this may result in areas of discoloration and residual oily material at the injection site beyond the withdrawal period.
Storage
Store at a temperature between 15° and 25°C. Once broached, contents should be used within 12 weeks.PRESENTATION: EXCEDE 100 Sterile Suspension is available in a 100 mL vial.
Zoetis is a trademark and Excede is a registered trademark of Zoetis or its licensors, used under license by Zoetis Canada Inc.
1Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Proposed Standard. CLSI Document M31-A (ISBN 1-56238-377-9). CLSI, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1832, 1999.
Zoetis Canada Inc., Kirkland QC H9H 4M7
30215400
1505-11-1
CPN: 1198348.2
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
