Combi-Pen-48
This treatment applies to the following species: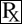 Company: Bimeda
Company: Bimeda
(penicillin G benzathine and penicillin G procaine Injectable Suspension)
For Subcutaneous Use In Beef Cattle Only
Antibiotic
300,000 units per mL
Combi-Pen-48 Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 065-506
Description
Combi-Pen-48 (penicillin G benzathine and penicillin G procaine Injectable Suspension) is available as an aqueous suspension in 100-mL, 250-mL and 500-mL multipledose vials. Each mL contains: Active Ingredients: 150,000 units penicillin G benzathine, 150,000 units penicillin G procaine. Inactive Ingredients: 11.7 mg Lecithin, 1.75 mg Sodium formaldehyde sulfoxylate, 1.20 mg Methylparaben, 0.14 mg Propylparaben, 8.19 mg Tween 40, 11.3 mg Span 40, 3.98 mg Sodium citrate (anhydrous), 20 mg Procaine hydrochloride, 1.04 mg Sodium carboxymethylcellulose, Water for Injection, q.s.
EACH mL OF SUSPENSION CONTAINS:
Active Ingredients
|
Penicillin G benzathine |
150,000 units |
|
Penicillin G procaine |
150,000 units |
Inactive Ingredients
|
Procaine hydrochloride |
20 mg |
|
Lecithin |
11.7 mg |
|
Span 40 |
11.3 mg |
|
Tween 40 |
8.19 mg |
|
Sodium citrate anhydrous |
3.98 mg |
|
Sodium formaldehyde sulfoxylate |
1.75 mg |
|
Methylparaben (as preservative) |
1.20 mg |
|
Sodium carboxymethylcellulose |
1.04 mg |
|
Propylparaben (as preservative) |
0.14 mg |
|
Water for Injection |
q.s. |
Not for Use In Humans
Keep Out of Reach of Children
ACTION: Penicillin G is an antibiotic which shows a marked bactericidal effect against certain organisms during their growth phase. It is relatively specific in its action against gram positive bacteria but is usually ineffective against gram-negative bacteria. It is normally recommended that any bacterial infection be treated as early as possible and with a dosage that will give effective blood levels. Although the recommended dosage of this product will give longer detectable penicillin blood levels than penicillin G procaine alone, it is recommended that a second dose be administered at 48 hours when treating a penicillin-susceptible bacterial infection.
The use of antibiotics in the management of disease is based on an accurate diagnosis and an adequate course of treatment. When properly used in the treatment of diseases caused by penicillin-susceptible organisms, most animals treated with the product will show a noticeable improvement within 24 to 48 hours. If improvement does not occur within this period of time, the diagnosis and course of treatment should be re-evaluated. It is recommended that the diagnosis and treatment of animal diseases be carried out by a veterinarian. Since many diseases look alike but require different types of treatment, the use of professional veterinary and laboratory services can reduce treatment time, costs and needless losses. Good housing, sanitation and nutrition are important in the maintenance of healthy animals and are essential in the treatment of disease.
 |
RESIDUE WARNING: Beef cattle should be withheld from slaughter for food use for thirty (30) days following last treatment. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal. |
 |
Combi-Pen-48 Indications
This product is indicated for the treatment of the following bacterial infections in beef cattle due to penicillin-susceptible microorganisms that are susceptible to the serum levels common to this particular dosage form, such as:
1. Bacterial Pneumonia (shipping fever complex) (Streptococcus spp., Actinomyces pyogenes, Staphylococcus aureus).
2. Upper Respiratory Infections such as rhinitis or pharyngitis (Actinomyces pyogenes).
3. Blackleg (Clostridium chauvoei).
Combi-Pen-48 Caution
Not for Use in Humans. Keep Out of Reach of Children.
Precautions
Exceeding the recommended doses and dosage levels may result in antibiotic residues beyond the withdrawal time. Do not inject this product intramuscularly. Penicillin G is a substance of low toxicity. However, side effects, or so-called allergic or anaphylactic reactions - sometimes fatal, have been known to occur in animals hypersensitive to penicillin and procaine. Such reactions can occur unpredictably with varying intensity. Animals administered penicillin G should be kept under close observation for at least one half hour. Should allergic or anaphylactic reactions occur, discontinue use of the product and immediately administer epinephrine following the manufacturer’s recommendations and call a veterinarian.As with all antibiotic preparations, use of this drug may result in overgrowth of non-susceptible organisms, including fungi. A lack of response by the treated animal, or the development of new signs or symptoms suggests that an overgrowth of non-susceptible organisms has occurred. In such instances, consult your veterinarian.
Since bactericidal drugs may interfere with the bacteriostatic action of tetracyclines, it is advisable to avoid giving penicillin in conjunction with tetracyclines.
ADMINISTRATION: The recommended dosage for beef cattle should be administered by subcutaneous injection only. Failure to use the subcutaneous route of administration may result in antibiotic residues in meat beyond the withdrawal time. Do not inject intramuscularly.
DOSAGE: Beef Cattle: 2 mL per 150 lb body weight given subcutaneously only (2000 units penicillin G procaine and 2000 units penicillin G benzathine per lb body weight). Treatment should be repeated in 48 hours.
IMPORTANT: Treatment in beef cattle must be limited to two (2) doses given by subcutaneous injection only.
Directions For Use
A thoroughly cleaned, sterile needle and syringe should be used for each injection (needles and syringes may be sterilized by boiling in water for 15 minutes).
Before withdrawing the solution from the bottle, disinfect the rubber cap on the bottle with a suitable disinfectant, such as 70 percent alcohol. The injection site should be similarly cleaned with the disinfectant. Use a 16 gauge needle, not more than 1 inch long. Use this product within 28 days of the first puncture and puncture a maximum of 15 times. Consider using automatic injection equipment or a repeater syringe if necessary to avoid exceeding 15 punctures. When using a draw-off spike or needle with bore diameter larger than 16 gauge, discard any product remaining in the vial immediately after use.
A subcutaneous injection should be made by pinching up a fold of the skin between the thumb and forefinger. The mid-neck region is the preferred injection site. Insert the needle under the fold in a direction approximately parallel to the surface of the body. When the needle is inserted in this manner, the medication will be delivered underneath the skin between the skin and the muscles. Proper restraint, such as the use of the chute and nose lead is needed for proper administration of the product.
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report suspected adverse drug events, for technical assistance or to obtain a copy of the SDS, contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
STORE BETWEEN 2°C - 8°C (36°F - 46°F). PROTECT FROM FREEZING. WARM TO ROOM TEMPERATURE AND SHAKE WELL BEFORE USING.
Dispose of containers in approved landfill or by incineration.
Manufactured for: Bimeda, Inc., LeSueur, MN 56058
www.bimeda.com
Combi-Pen-48 is a Registered Trademark of Bimeda, Inc.
8BEN301
|
100 mL (3.38 fl oz) Sterile, Multiple Dose Vial |
1COM301 |
8COM301 Rev. 05/22 |
|
250 mL (8.45 fl oz) Sterile, Multiple Dose Vial |
1COM302 |
8COM305 Rev. 05/22 |
|
500 mL (16.91 fl oz) Sterile, Multiple Dose Vial |
1COM303 |
8COM308 Rev. 05/22 |
CPN: 1399064.10
Div. Cross Vetpharm Group, Ltd.
475 NORTH MARTINGALE ROAD, SUITE 1200, SCHAUMBURG, IL, 60173
| Telephone: | 630-928-0361 | |
| Fax: | 630-928-0362 | |
| Website: | www.bimedaus.com | |
| Email: | us-sales@bimeda.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
