Bovicam 20 (Canada)
This treatment applies to the following species: Company: Modern Veterinary Therapeutics
Company: Modern Veterinary Therapeutics
Meloxicam Injection
Meloxicam 20 mg/mL
Sterile
Veterinary Use Only
DIN 02527871
Each mL contains 20 mg meloxicam and 150 mg ethanol, as preservative.
Description
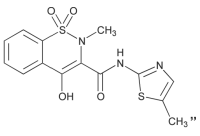
4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide, Empirical formula: C14H13N3O4S2. Molecular weight: 351.40 g/mol. Chemical structure:
Therapeutic Classification: Nonsteroidal anti-inflammatory drug (NSAID).
Bovicam 20 Indications
Cattle: As an aid in improving appetite and weight gains when administered at the onset of diarrhea, in combination with oral rehydration therapy, in calves over one week of age.
For relief of pain following de-budding of horn buds in calves less than 3 months of age.
For the symptomatic treatment of inflammation and pain associated with acute clinical mastitis.
For the reduction of pain associated with abdominal surgery such as caesarean section.
Sheep: For single dose use in sheep and lambs 14 days of age or older for the alleviation of pain and inflammation.
Swine: For use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation.
Dosage and Administration
Cattle: Single subcutaneous or intravenous injection of 0.5 mg meloxicam/kg body weight (2.5 mL/100 kg). For reduction in pain associated with abdominal surgery, administer 10 to 20 minutes before the painful procedure.
Sheep: Single subcutaneous injection administered 15-30 minutes prior to painful procedure. Inject the product high on the neck behind the ear at a dose of 1.0 mg meloxicam/kg body weight (1.0 mL/20 kg).
Swine: Single intramuscular injection at a dosage of 0.4 mg meloxicam/kg body weight (2.0 mL/100 kg). If required, a second administration of meloxicam can be given after 24 hours.
A multi-dosing injection system should be used if more than 10 punctures are anticipated.
Contraindications
Do not use in animals suffering from gastrointestinal disorders such as irritation and hemorrhage, impaired hepatic, cardiac or renal function and hemorrhagic disorders, or where there is individual hypersensitivity to the product.
Do not administer concurrently with steroidal, other nonsteroidal anti-inflammatory drugs or with anti-coagulant agents.
Concomitant use of NSAIDs with aminoglycoside antimicrobials in very young animals may result in renal toxicity.
Cautions: Do not use in bulls, rams or boars intended for breeding.
Avoid use in very severely dehydrated, hypovolaemic or hypotensive animals which require parenteral rehydration, as there may be a potential risk of increased renal toxicity.
Use of anti-inflammatories in very young or debilitated animals may involve additional risk.
The safety of meloxicam has not been evaluated in lambs less than 14 days of age.
The safety of meloxicam in breeding and pregnant sows has not been fully evaluated.
Available data suggest that meloxicam has no harmful effects in the second and third trimester of pregnancy. However, the potential effects of meloxicam on imminent parturition have not been evaluated. This product can be used in lactating sows.
Treatment of cows with meloxicam before abdominal surgery reduces post-operative pain. An anti-inflammatory alone will not provide adequate pain relief during the surgical procedure. To obtain adequate pain relief during surgery, co-medication with an appropriate analgesic is recommended.
Warnings
- Treated animals must not be slaughtered for use in food for at least 20 days for cattle, 5 days for swine, and 11 days for sheep after the latest treatment with this drug.
- Milk taken from treated cows during treatment and within 96 hours after the latest treatment with this drug must not be used in food.
- Do not use in lactating ewes or pregnant ewes within 11 days of lambing where milk may be used or processed for human consumption.
- Do not use in calves to be processed for veal as a withdrawal period has not been established for pre-ruminating calves.
- People with known hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) should not handle this product.
- Caution should be taken to avoid accidental self-injection, ingestion and contact with eyes.
- This product can cause eye irritation. In case of contact with the eyes, immediately rinse thoroughly with water.
- Keep out of the reach of children.
Adverse Reactions
Transient, slight injection site swellings may occasionally be seen following subcutaneous injection and intravenous injection.In very rare cases, anaphylactoid reactions may occur and should be treated symptomatically.
Pharmacology
Meloxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class which acts by inhibition of prostaglandin synthesis, thereby exerting anti-inflammatory, analgesic and anti-pyretic properties. Meloxicam also has anti-endotoxic properties because it has been shown to inhibit production of thromboxane B2 induced by E. coli endotoxin administration in calves.
Toxicology: The acute oral toxicity (LD50) of meloxicam assessed in rats, mini-pigs, mice and rabbits was >80 mg/kg, 1600 mg/kg, 470 mg/kg and 320 mg/kg, respectively. Repeated dose toxicity studies in rats, mice and mini-pigs demonstrated that the primary target organs for toxicity were the gastrointestinal tract (pyloric, duodenal and small intestine ulceration) and kidneys (scarring, necrosis and pyelonephritis).
Storage
Store between 15°C to 25°C. Keep from freezing. Discard 28 days from first opening.Presentation: Bovicam 20 is available in 50 mL and 100 mL multi-dose vials.
Manufactured for:
Modern Veterinary Therapeutics, LLC, Sunrise, Florida 33326 USA
info@modernveterinarytherapeutics.com
www.modernveterinarytherapeutics.com
Imported by:
Modern Veterinary Therapeutics Inc, 261065 Wagon Wheel Way, Bay 3, Balzac (Rocky View County), AB T4A 0T5
Orders & Product information: Call 1 888 590-9839
Revision Date: 05/24
E5447-S.0524
CPN: 1354033.1
261065 WAGON WHEEL WAY, ROCKY VIEW COUNTY, AB, T4A 0T5
| Telephone: | 407-852-8039 | |
| Toll-Free: | 888-590-9839 | |
| Website: | www.modernveterinarytherapeutics.com | |
| Email: | info@modernveterinarytherapeutics.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
