3FLEX
This treatment applies to the following species:Porcine Reproductive & Respiratory Syndrome-Circovirus Vaccine
Reproductive & Respiratory Form, Type 2, Modified Live Virus, Killed Baculovirus Vector
Mycoplasma Hyopneumoniae Bacterin
This product has been shown to be effective for the vaccination of healthy swine 3 weeks of age or older against Porcine Circovirus Type 2 (PCV2), Mycoplasma hyopneumoniae, and respiratory form of Porcine Reproductive and Respiratory Syndrome (PRRS) virus. The duration of immunity is at least 4 months against the respiratory form of PRRS disease, at least 4 months against PCV2, and at least 26 weeks against Mycoplasma hyopneumoniae. For more information regarding efficacy and safety data go to productdata.aphis.usda.gov.
Composition
This product contains PCV2a.Precautions
Store at 35-46°F (2-8°C). Do not freeze. Use entire contents when first opened. Do not vaccinate within 21 days before slaughter. Do not mix with other products, except as specified on the carton. In case of anaphylactoid reaction, administer epinephrine. Not for use in pregnant swine or boars. Indicated for use in PRRS virus-positive herds only. Vaccine virus may be shed and transmitted to other populations of swine in contact with vaccinated swine. The duration of potential vaccine virus transmission may vary. Use of the vaccine in herds intended to remain PRRS virus seronegative is contraindicated. Introduction of vaccinated pigs into herds intended to remain PRRS virus seronegative is contraindicated. In case of human exposure, contact a physician. Inactivate unused contents before disposal.Preservative: Neomycin.
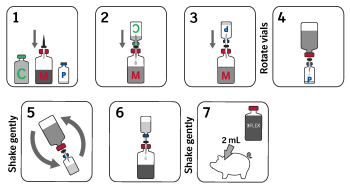
Directions and dosage: Shake each bottle of liquid product before use. Insert a vented transfer spike into Ingelvac MycoFLEX® 2 headspace bottle. Invert the Ingelvac CircoFLEX® 1 bottle over the Ingelvac MycoFLEX® 2. Transfer the full contents of the vaccine into the Ingelvac MycoFLEX® 2 bottle via the transfer spike and gently shake. Remove the Ingelvac CircoFLEX® 1 bottle and slowly insert the transfer spike into the Ingelvac PRRS® MLV 3 vaccine bottle, releasing the vacuum. Transfer a portion of the mixed vaccine from the Ingelvac MycoFLEX® 2 bottle into the Ingelvac PRRS® MLV 3 bottle. The amount transferred needs to be enough to rehydrate the Ingelvac PRRS® MLV 3 product. Flip the Ingelvac PRRS® MLV 3 and Ingelvac MycoFLEX® 2 bottles - still attached by transfer spike - to transfer rehydrated product back into the Ingelvac MycoFLEX® 2 bottle. Shake well and use immediately. Rehydrate only with the accompanying vaccines, do not mix other materials.
Using aseptic technique, inject a single 2 mL dose of vaccine intramuscularly.
3FLEX®, Ingelvac MycoFLEX®, Ingelvac CircoFLEX®, and Ingelvac PRRS® are registered trademarks of Boehringer Ingelheim Vetmedica GmbH. Used under license.
Boehringer Ingelheim Animal Health USA Inc., St. Joseph, MO 64506
Phone: 1 (888) 637-4251
VLN/PCN 124/49K9.R0
|
50 Doses HSB Mix to 100 mL |
This package contains one 50 dose vial of Ingelvac CircoFLEX® 1, one 50 dose vial of Ingelvac MycoFLEX® 2, and one 50 dose vial of Ingelvac PRRS® MLV 3. The Ingelvac MycoFLEX® 2 is supplied in a headspace bottle (HSB) with extra capacity for the purpose of aseptic mixing. |
129903-06 |
|
250 Doses HSB Mix to 500 mL |
This package contains one 250 dose vial of Ingelvac CircoFLEX® 1, one 250 dose vial of Ingelvac MycoFLEX® 2, and one 250 dose vial of Ingelvac PRRS® MLV 3. The Ingelvac MycoFLEX® 2 is supplied in a headspace bottle (HSB) with extra capacity for the purpose of aseptic mixing. |
129910-02 |
CPN: 1028266.3
3239 SATELLITE BLVD., BLDG 500, DULUTH, GA, 30096
| Telephone: | 800-325-9167 | |
| Customer Service: | 888-637-4251 | |
| Technical Service: | 888-637-4251 | |
| Fax: | 816-236-2717 | |
| Website: | www.boehringer-ingelheim.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
