Cardioxane Injection: Package Insert / Prescribing Info
Package insert / product label
Generic name: dexrazoxane
Dosage form: injection, powder, for solution
Drug class: Miscellaneous uncategorized agents
Medically reviewed by Drugs.com. Last updated on Jun 16, 2025.
IMPORTANT DRUG INFORMATION
Subject: Temporary Importation of Cardioxane 500 mg Powder for Solution for Infusion (dexrazoxane hydrochloride) to address drug shortage issues
Dear Healthcare Professional,
|
Important Reconstitution Note for Medication Error Prevention: Prescribers and pharmacists must be alert to the differences between the FDA-approved dexrazoxane products and Cardioxane’s presentation and reconstitution and dilution instructions in order to prevent medication errors. Appropriate quality assurance measures should be enacted to reduce the risk of medication errors. See comparison table below for more information. Cardioxane has a different vial capacity than FDA-approved dexrazoxane products, and, therefore must be reconstituted with a different volume of Water for Injection. Cardioxane 500 mg Powder for Solution for Infusion is provided in a vial with a capacity of 25 mL. To reconstitute, add 25 mL Water for Injection (USP) to the vial. The concentration of the resulting reconstitution solution will be 20 mg/mL – this solution should not be stored and must be further diluted before administration to the patient. Further dilute the reconstitution solution with Lactated Ringer's Injection, USP to a final concentration between 1.3 and 3 mg/mL. The diluted infusion solution should be used immediately or stored for a maximum of 4 hours under refrigeration. |
Due to the current critical shortage of Dexrazoxane for Injection, 250 mg/vial and 500 mg/vial in the United States (U.S.) market, Clinigen Healthcare Ltd is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of Dexrazoxane for Injection 500 mg/vial.
Clinigen Healthcare Ltd has initiated temporary importation into the U.S. of Cardioxane 500 mg Powder for Solution for Infusion (dexrazoxane hydrochloride), a non-FDA approved product, to help alleviate the shortage. The Cardioxane 500 mg Powder for Solution for Infusion was manufactured by Cenexi – Laboratoires Thissen S.A. in its FDA-inspected facility in Braine l’Alleud, Belgium and is licensed for sale in the United Kingdom. Clinigen Healthcare Ltd’s distributor for Cardioxane in the US is Cumberland Pharmaceuticals Inc.
At this time, no other entities except Clinigen Healthcare Ltd and its distributor Cumberland Pharmaceutical Inc. are authorized by the FDA to import or distribute Cardioxane 500 mg Powder for Solution for Infusion in the U.S. This does not constitute an FDA approval for the Cardioxane 500 mg Powder for Solution for Infusion being distributed in the U.S.
Cardioxane 500 mg Powder for Solution for Infusion contains the same active substance, dexrazoxane hydrochloride, as FDA-approved Zinecard. It is available as 500 mg/vial (no 250 mg/vial presentation is available). It is a clinically acceptable substitute for the out of stock U.S. Dexrazoxane for Injection products. Cardioxane is available only by prescription in the U.S.
In the U.S., the labelling for Dexrazoxane Injection (Zinecard) includes the following indication: ZINECARD is indicated for reducing the incidence and severity of cardiomyopathy associated with doxorubicin administration in women with metastatic breast cancer who have received a cumulative doxorubicin dose of 300 mg/m2 and who will continue to receive doxorubicin therapy to maintain tumor control. Do not use with the initiation of doxorubicin therapy.
Comparison of Cardioxane and FDA-approved Zinecard
It is important to note the following differences between the U.S. FDA approved Dexrazoxane for Injection products and Cardioxane (a comparison of the carton and vial labels is appended to this letter for reference):
|
Dexrazoxane for Injection (U.S.) |
Cardioxane (UK) |
Action to Take |
|
Packaged with complete prescribing Information. |
Packaged with a Patient Information Leaflet, with information for Healthcare Professionals – this does not provide complete information for use. |
Please refer to the package insert for the U.S. FDA approved Zinecard (dexrazoxane) for Injection for full prescribing information. |
|
Presentation 250 mg/vial and 500 mg/vial. |
Presentation 500 mg/vial only. |
Prescribers and pharmacists must be alert to this difference in presentation in order to prevent medication errors. |
|
Zinecard 500 mg/vial is reconstituted with 50 mL of Sterile Water for Injection USP. Reconstituted solution has a concentration of 10 mg/mL. |
Cardioxane 500mg/vial is reconstituted with 25 mL of Water for Injection. Reconstituted solution has a concentration of 20 mg/mL. |
The Cardioxane reconstituted solution will have a concentration of 20 mg/mL, this must be considered when further diluting to give an infusion solution. Prescribers and pharmacists must be alert to this difference in order to prevent medication errors. |
|
Following reconstitution with Sterile Water for Injection, USP, Zinecard is stable for 30 minutes at room temperature or if storage is necessary, up to 3 hours from the time of reconstitution when stored under refrigeration, 2° to 8°C (36° to 46°F). |
No storage recommendations are given for the reconstituted solution – it should be immediately diluted for infusion. |
Use the reconstituted solution immediately. |
|
The reconstituted solution is diluted further with Lactated Ringer's Injection, USP to a concentration of 1.3 mg/mL to 3 mg/mL in intravenous infusion bags for intravenous infusion. |
The reconstituted solution is diluted further with Lactated Ringer’s Injection or Sodium Lactate 0.16 M to a concentration between 4 mg/mL to 10 mg/mL. |
Recall that the reconstitution solution will have a concentration of 20 mg/mL. Follow the instructions given within the Zinecard Prescribing Information – dilute with Lactated Ringer’s Injection USP to 1.3 mg/mL to 3 mg/mL in intravenous infusion bags for intravenous infusion. |
|
The diluted infusion solutions are stable for one hour at room temperature or if storage is necessary, up to 4 hours when stored under refrigeration, 2° to 8°C (36° to 46°F). |
Reconstituted and subsequently diluted product should be used immediately or within 4 hours if stored between 2°C and 8°C. |
The diluted infusion solution should be used immediately or stored for a maximum of 4 hours under refrigeration, 2° to 8°C (36° to 46°F). |
Please note that no barcode appears on the carton for Cardioxane 500 mg Powder for Solution for Infusion, therefore it will not be appropriately recognized by scanning systems used in the United States. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
To order Cardioxane 500 mg Powder for Solution for Infusion, please contact the distributor Cumberland Pharmaceuticals Inc., at 615-255-0068, or by email at jherman@cumberlandpharma.com.
To report adverse events or other safety-related information, including medication errors to Clinigen Healthcare, you may complete the available form at http://www.clinigengroup.com/node/99 and send via email to patientsafety@clinigengroup.com or fax to 1-800-861-9362. Adverse events and medication errors, or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, or regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
We urge you to contact our Medical Information Department at toll-free 888-758-7954 during office hours (Monday to Friday, 8am to 5:30pm Eastern Time), or at 913-339-8424 out of office hours, or email clinigenUS@medinfodept.com, if you have any questions about the information contained in this letter or the safe and effective use of Cardioxane 500 mg Powder for Solution for Infusion.
Comparison of Carton and Vial Labels (Zinecard and Cardioxane 500 mg Powder for Solution for Infusion)
|
Item |
Zinecard (US) |
Cardioxane 500 mg Powder for Solution for Infusion |
|
Carton Label |
Principal Panel NDC 0013-8727-89 Single-Dose Vial Zinecard® (dexrazoxane) for injection 500 mg* Sterile, Pyrogen-Free Lyophilizate For Intravenous Use Only Pfizer Injectables Rx only Side Panel 1 Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Upon reconstitution with 50 mL of Sterile Water for Injection, USP, the pH of the resultant solution is 1.0 to 3.0. Reconstituted solutions are stable for 30 minutes at room temperature or if storage is necessary, up to 3 hours from the time of reconstitution when stored under refrigeration, 2° to 8°C (36° to 46°F). Further dilution with Lactated Ringer’s, USP, is necessary prior to administration via IV drop. DO NOT ADMINISTER RECONSTITUTED SOLUTION DIRECTLY. Discard unused solutions. DOSAGE AND USE: See accompanying prescribing information. *Each vial contains dexrazoxane hydrochloride equivalent to 500 mg dexrazoxane. The pH is adjusted with Hydrochloric Acid, NF. Side Panel 2 Barcode MADE IN ITALY Distributed by Pharmacia & Upjohn Co Division of Pfizer Inc NY, NY 10017 |
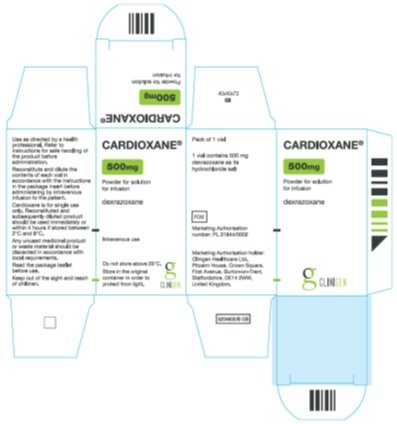
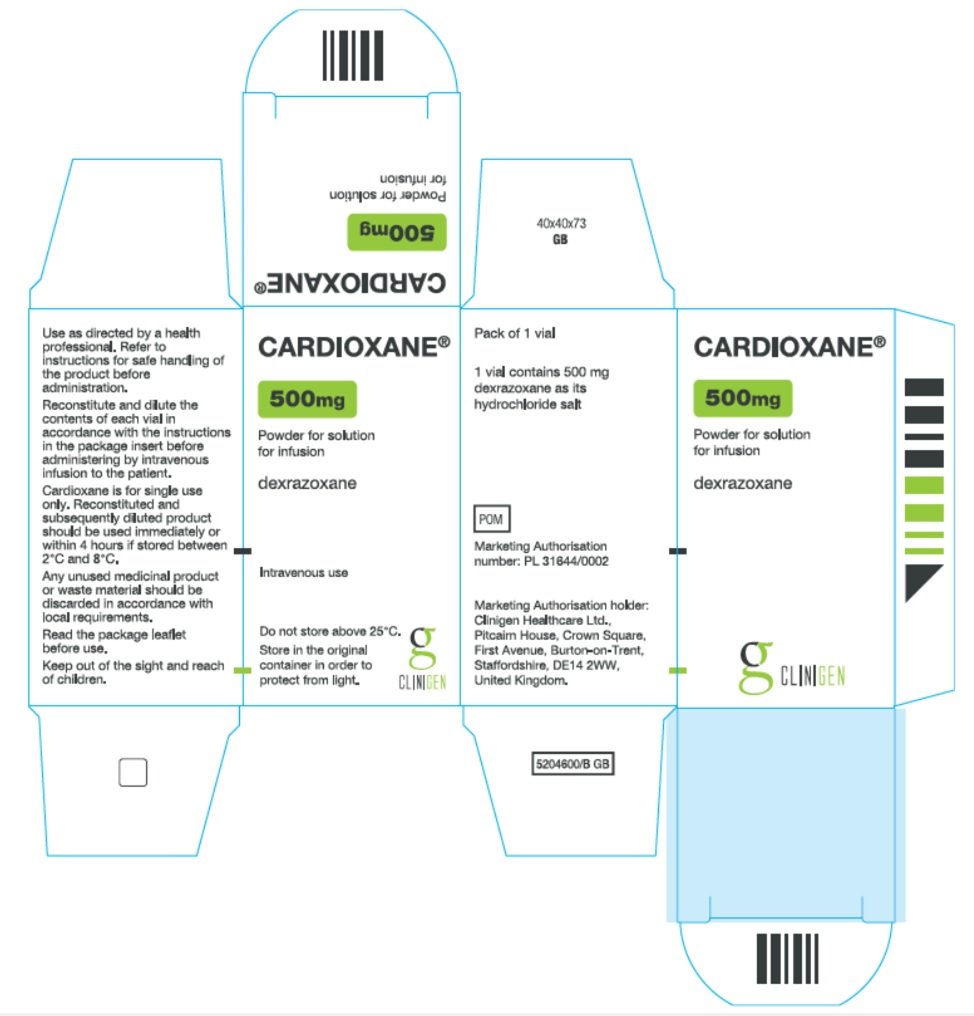
Principal Panel Cardioxane® 500mg Powder for solution for infusion Dexrazoxane Intravenous use Do not store above 25°C. Store in the original container in order to protect from light. Side Panel 1 Use as directed by a health professional. Refer to instructions for safe handling of product before administration. Reconstitute and dilute the contents of each vial in accordance with the instructions in the package insert before administering by intravenous infusion to the patient. Cardioxane is for single use only. Reconstituted and subsequently diluted product should be used immediately or within 4 hours if stored between 2°C and 8°C. Any unused medicinal product should be discarded in accordance with local requirements. Read the package leaflet before use. Keep out of the sight and reach of children.
Pack of 1 vial 1 vial contains 500 mg dexrazoxane as its hydrochloride salt POM Marketing Authorisation number: PL 31644/0002 Marketing Authorisation holder: Clinigen Healthcare Ltd., Pitcairn House, Crown Square, First Avenue, Burton-on-Trent, Staffordshire, DE14 2WW, United Kingdom. |
|
Carton Label Image |
|
|
|
Item |
Zinecard (US) |
Cardioxane 500 mg Powder for Solution for Infusion |
|
Vial Label |
Principal Panel NDC 0013-8727-89 Single-Dose Vial Zinecard® (dexrazoxane) for injection 500 mg* Sterile, Pyrogen-Free Lyophilizate For Intravenous Use Only Rx only Side Panel
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Upon reconstitution with 50 mL of Sterile Water for Injection, USP, the pH of the resultant solution is 1.0 to 3.0. Reconstituted solutions are stable for 30 minutes at room temperature or if storage is necessary, up to 3 hours from the time of reconstitution when stored under refrigeration, 2° to 8°C (36° to 46° F). Further dilution with Lactated Ringer’s, USP, is necessary prior to administration via IV drop. DO NOT ADMINISTER RECONSTITUTED SOLUTION DIRECTLY. Discard unused solutions. DOSAGE AND USE: See accompanying prescribing information. Distributed by Pharmacia & Upjohn Co Division of Pfizer Inc NY, NY 10017 MADE IN ITALY LOT EXP |
Principal Panel Cardioxane® 500 mg Powder for Solution for Infusion Dexrazoxane (as its hydrochloride salt) Intravenous use for short infusion. Do not store above 25°C. Store in the original container. Protect from direct sunlight. Marketing Authorisation number: PL 31644/0002 Keep out of the reach and sight of children. POM Side Panel Clinigen Healthcare Ltd. Pitcairn House, First Avenue, Burton-on-Trent, Staffordshire DE14 2WW, UK. |
|
Vial Label Image |
|
|
| CARDIOXANE
dexrazoxane injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Clinigen Healthcare Ltd (856162487) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cenexi - Laboratoires Thissen Sa | 370088959 | manufacture(76310-002) , label(76310-002) | |
More about dexrazoxane
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous uncategorized agents
- Breastfeeding
- En español