Zusduri: Package Insert / Prescribing Info
Package insert / product label
Generic name: mitomycin
Dosage form: kit
Drug class: Antibiotics / antineoplastics
J Code (medical billing code): J9999 (Not otherwise classified, antineoplastic drugs)
Medically reviewed by Drugs.com. Last updated on Jul 8, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ZUSDURI™ (mitomycin) for intravesical solution
Initial U.S. Approval: 1974
Indications and Usage for Zusduri
ZUSDURI is an alkylating drug indicated for the treatment of adult patients with recurrent low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC). (1)
Zusduri Dosage and Administration
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- Risks in Patients with Perforated Bladder: Evaluate the bladder before the intravesical instillation of ZUSDURI. Do not administer to patients with a perforated bladder or in whom the integrity of the bladder mucosa has been compromised. (4, 5.1).
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and to use effective contraception. (5.2, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common (≥ 10%) adverse reactions, including laboratory abnormalities, that occurred in patients were increased creatinine, increased potassium, dysuria, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, increased eosinophils, decreased lymphocytes, urinary tract infection, decreased neutrophils, and hematuria. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact UroGen Pharma at 1-855-987-6436 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2025
Full Prescribing Information
1. Indications and Usage for Zusduri
ZUSDURI™ is indicated for the treatment of adult patients with recurrent low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC).
2. Zusduri Dosage and Administration
2.1 Important Administration Instructions
Administer ZUSDURI by intravesical instillation only. Do not administer by pyelocalyceal instillation or by any other route.
2.2 Recommended Dose
The recommended dose of ZUSDURI is 75 mg (56 mL) instilled once weekly for six weeks into the bladder via a urinary catheter. [see Dosage and Administration (2.4)]
2.3 Preparation Instructions
See the Instructions for Pharmacy enclosed in the carton for complete information on preparation.
ZUSDURI must be reconstituted with sterile hydrogel under chilled conditions. Reconstituted ZUSDURI has reverse thermal properties with a gelation point of approximately 19°C (66°F) and will appear as a viscous liquid under chilled conditions and a semisolid gel at room temperature.
Storage Instructions for Reconstituted ZUSDURI:
- Instill reconstituted ZUSDURI as soon as possible.
- If not used immediately, store reconstituted ZUSDURI:
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 7 days; or
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
- Discard 7 days after reconstitution.
- Protect from light.
- Avoid excessive heat over 40°C (104°F).
ZUSDURI is a hazardous drug. Follow applicable special handling and disposal procedures.1
2.4 Bladder Instillation of ZUSDURI
See the Instructions for Administration enclosed in the carton for complete information on bladder instillation.
ZUSDURI must be chilled at -3°C to 5°C (27°F to 41°F) to convert to a viscous liquid prior to instillation. When instilling ZUSDURI, each syringe must be emptied within thirty (30) seconds to avoid gelation.
Instillation of ZUSDURI requires syringes and a urinary catheter with fixed Luer Lock connectors.
Advise patients that ZUSDURI may discolor urine to a violet to blue color following the instillation procedure. Advise patients for at least 24 hours post-instillation to avoid urine contact with skin, to void urine sitting on a toilet, to wash hands and genital area with water and soap after each urination, and to flush the toilet several times after use.
3. Dosage Forms and Strengths
For intravesical solution: A kit containing the following:
- Two 40 mg (each) single-dose vials of sterile, lyophilized, grey to greyish-purple, cake or powder of mitomycin for intravesical solution.
- One single-dose vial of 60 mL of sterile, clear, colorless gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), to be used as a vehicle for reconstitution.
4. Contraindications
ZUSDURI is contraindicated in patients with:
- Perforation of the bladder [see Warnings and Precautions (5.1)],
- Prior hypersensitivity reactions to mitomycin or any component of the product.
5. Warnings and Precautions
5.1 Risks in Patients with Perforated Bladder
ZUSDURI may lead to systemic exposure to mitomycin and severe adverse reactions if administered to patients with a perforated bladder or to those in whom the integrity of the bladder mucosa has been compromised.
Evaluate the bladder before the intravesical instillation of ZUSDURI and do not administer to patients with a perforated bladder or mucosal compromise until bladder integrity has been restored [see Contraindications (4)].
5.2 Embryo-Fetal Toxicity
Based on findings in animals and mechanism of action, ZUSDURI can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of mitomycin resulted in teratogenicity. Advise females of reproductive potential to use effective contraception during treatment with ZUSDURI and for 6 months following the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ZUSDURI and for 3 months following the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
The safety of ZUSDURI was evaluated in ENVISION, a single-arm, multicenter study in 240 patients with recurrent LG-IR-NMIBC [see Clinical Studies (14)]. Patients received 75 mg ZUSDURI instilled once a week for 6 consecutive weeks. The median number of doses of ZUSDURI administered to patients was 6 (range 1-6) doses and 228 patients (95%) received all six scheduled doses.
Serious adverse reactions occurred in 12% of patients who received ZUSDURI, including urinary retention (0.8%) and urethral stenosis (0.4%). A fatal adverse reaction of cardiac failure occurred in 1 patient (0.4%) receiving ZUSDURI.
Permanent discontinuation of ZUSDURI due to an adverse reaction occurred in 2.9% of patients, including 1.7% who discontinued due to a renal or urinary disorder.
Dosage interruption of ZUSDURI due to adverse reactions occurred in 10% of patients. Adverse reactions (≥ 2%) which required dosage interruption were urinary tract infection (2.5%) and dysuria (2.5%).
The most common (≥ 10%) adverse reactions, including laboratory abnormalities, that occurred in patients were increased creatinine, increased potassium, dysuria, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, increased eosinophils, decreased lymphocytes, urinary tract infection, decreased neutrophils, and hematuria.
Table 1 summarizes the adverse reactions in ENVISION.
| Adverse Reaction | ZUSDURI N = 240 |
|
|---|---|---|
| All Grades (%) | Grade 3 or 4†
(%) |
|
| Renal and urinary disorders | ||
| Dysuria | 23 | 0.4 |
| Hematuria‡ | 10 | 0 |
| Infections and infestations | ||
| Urinary tract infection‡ | 12 | 0.8 |
Clinically relevant adverse reactions occurring in < 10% of patients receiving ZUSDURI in ENVISION included increased urinary frequency, fatigue, urinary incontinence, urinary retention, urethral stenosis, genital pain, urinary urgency, genital edema, genital pruritus, genital rash, urethritis, acute kidney injury, balanoposthitis, and nocturia.
Table 2 summarizes laboratory abnormalities in ENVISION.
| Laboratory Abnormality | ZUSDURI† | |
|---|---|---|
| All Grades (%) | Grade 3 or 4‡
(%) |
|
|
||
| Hematology | ||
| Hemoglobin Decreased | 17 | 0.8 |
| Eosinophils Increased | 15 | 0 |
| Lymphocytes Decreased | 14 | 0.4 |
| Neutrophils Decreased | 10 | 0.4 |
| Chemistry | ||
| Creatinine Increased | 29 | 1.3 |
| Potassium Increased | 26 | 2.2 |
| Aspartate Aminotransferase (AST) Increased | 15 | 0.4 |
| Alanine Aminotransferase (ALT) Increased | 15 | 0.4 |
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on findings in animals and mechanism of action, ZUSDURI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on ZUSDURI use in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of mitomycin resulted in teratogenicity (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% - 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of mitomycin in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with ZUSDURI and for 1 week following the last dose.
8.3 Females and Males of Reproductive Potential
ZUSDURI can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating ZUSDURI.
8.5 Geriatric Use
Of the 240 patients receiving ZUSDURI in the ENVISION study, 162 (68%) were 65 years of age and older and 89 (37%) were 75 years of age and older. No significant overall differences in safety or efficacy were observed in patients 65 years of age and older, and in patients 75 years of age and older, compared to younger patients.
8.6 Renal Impairment
Avoid use of ZUSDURI in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] < 30 mL/min). A higher incidence of hematuria and urinary tract infections was observed in patients with moderate renal impairment (eGFR 30 to <60 mL/min). Monitor patients with moderate renal impairment for increased adverse reactions. No dosage adjustments are recommended in patients with mild (eGFR 60 to <90 mL/min) or moderate renal impairment.
11. Zusduri Description
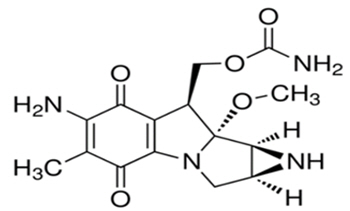
Mitomycin (also known as mitomycin-C) is an alkylating drug isolated from the broth of Streptomyces. Mitomycin is a blue-violet crystalline powder with a molecular formula of C15H18N4O5, and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane, and it has the following structural formula:
Mitomycin is heat stable, has a high melting point, and is freely soluble in organic solvents.
ZUSDURI is supplied in a kit containing two vials of sterile lyophilized mitomycin for intravesical solution, 40 mg each, and one vial of 60 mL of sterile hydrogel, to be used as a vehicle for reconstitution.
Mitomycin for intravesical solution is a sterile, lyophilized, grey to greyish-purple, cake or powder that contains mitomycin 40 mg and mannitol 80 mg in each vial.
Hydrogel is a sterile, clear, colorless gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), which contains 0.11 g hydroxypropyl methylcellulose, 17.12 g poloxamer, 0.63 g polyethylene glycol, and water for injection in each vial.
Once reconstituted, ZUSDURI is a clear, purple, viscous liquid at 2°C to 8°C (36°F to 46°F) or semisolid gel at room temperature, which may contain a few visible particles and have a pH between 6.0 and 8.0.
12. Zusduri - Clinical Pharmacology
12.1 Mechanism of Action
Mitomycin inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of mitomycin-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed.
12.2 Pharmacodynamics
Mitomycin exposure-response relationships and time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The systemic exposure of mitomycin following instillation of 75 mg of mitomycin as ZUSDURI into the bladder was evaluated pre-instillation and hourly for up to six hours post-instillation in six patients. Mitomycin mean (range) maximum concentration (Cmax) is 2.3 ng/mL (0.2 to 8.9 ng/mL), which is less than 1% of the expected Cmax after intravenous administration.
Metabolism
Mitomycin is metabolized primarily in the liver, but metabolism occurs in other tissues as well. It is believed that the rate of clearance is inversely proportional to the maximal serum concentration because of saturation of the degradative pathways.
Excretion
Following instillation into the bladder, ZUSDURI forms a semisolid gel which dissolves in the urine. Patients reported visible gel in urine for up to 24 hours (median 5 hours) after instillation. Mitomycin is excreted unchanged in the urine. Systemically absorbed mitomycin is rapidly cleared from the serum and approximately 10% is excreted unchanged in the urine.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate long-term studies in animals to evaluate carcinogenic potential from instillation of mitomycin into the bladder have not been conducted. Mitomycin has been found to be carcinogenic in rats and mice. At doses approximating the recommended intravenous clinical dose in humans, mitomycin produced a greater than 100% increase in tumor incidence in male Sprague-Dawley rats, and a greater than 50% increase in tumor incidence in female Swiss mice.
The effect of ZUSDURI on fertility is unknown.
14. Clinical Studies
ENVISION Study
The efficacy of ZUSDURI was evaluated in ENVISION (NCT05243550), a single-arm, multicenter trial in 240 adults with recurrent low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC), of whom 223 were evaluable for response.
LG-IR-NMIBC was defined as Ta disease, histologically confirmed by biopsy, having one or two of the following: the presence of multiple tumors, a solitary tumor > 3 cm, and/or early or frequent recurrence (≥ 1 occurrence of LG-NMIBC within 1 year of the current diagnosis). Patients were required to have a previous occurrence of LG-NMIBC (Ta) treated by TURBT. The trial excluded patients with T1 tumors, or history of high-grade NMIBC within the previous two years, and/or those with prior intravesical chemotherapy within the prior two years (except for a single dose of intravesical chemotherapy immediately after any previous TURBT) and/or Bacillus Calmette-Guerin treatment within the previous year.
Patients received 75 mg ZUSDURI via urinary catheter once a week for 6 weeks.
Assessment of tumor status was performed every 3 months by cystoscopy, for-cause biopsy, and urine cytology. The major efficacy outcome measures were complete response rate (CR) at 3 months (defined as no detectable disease in the bladder by cystoscopy, biopsy [if indicated], and urine cytology) and duration of response.
The median age of patients was 70 years (range, 30-92 years); 62% were male; race was White (97.8%), Black (0.9%), Asian (0.9%), or not reported (0.4%); 1.3% were Hispanic/Latino. Multiple tumors were present in 84% of patients, 6% had a tumor > 3 cm, 55% had a previous LG-NMIBC occurrence within 1 year of the current diagnosis, and all patients had a prior TURBT for LG-NMIBC.
Efficacy results are summarized in Table 3.
| Efficacy Outcome Measure | ZUSDURI N = 223 |
|---|---|
| + Denotes ongoing response. | |
|
|
| Complete Response Rate %
(95% CI) | 78% (72, 83) |
| Duration of Response* | |
| Range in months | (0.0, 25.0+) |
| % (n) with duration ≥ 12 months | 79% (137) |
16. How is Zusduri supplied
How Supplied
ZUSDURI is available in a kit (NDC 72493-106-03) containing the following:
- Two 40 mg (each) single-dose vials of mitomycin for intravesical solution supplied as a sterile, lyophilized, grey to greyish-purple, cake or powder. (NDC 72493-104-40)
- One single-dose vial of 60 mL sterile hydrogel supplied as a sterile, clear, colorless gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), to be used as a vehicle for reconstitution. (NDC 72493-105-60)
Storage and Handling
Store ZUSDURI at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Avoid excessive heat over 40°C (104°F). Protect from light.
ZUSDURI is a hazardous drug. Follow applicable special handling and disposal procedures.1
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare providers of a known or suspected pregnancy [see Warnings and Precautions (5.2) and Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with ZUSDURI and for 6 months following the last dose [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ZUSDURI and for 3 months following the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with ZUSDURI and for 1 week following the last dose [see Use in Specific Populations (8.2)].
Important Post-Treatment Instructions [see Dosage and Administration (2.4)]
Advise patients that ZUSDURI contains mitomycin which is a violet to blue color and may discolor urine following the instillation procedure.
Advise patients to avoid contact with urine for at least 24 hours post-instillation [see Clinical Pharmacology (12.3)].
Advise patients to avoid urine contact with skin by voiding sitting on a toilet, flushing the toilet several times after use, and to wash hands, perineum or glans with soap and water after each instillation procedure.
Advise patients to wash clothing soiled with urine promptly and separately from other clothing.
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540
ZUSDURI™ is a trademark and UroGen® is a registered trademark of UroGen Pharma, Ltd.
U.S. Patent Nos. 9,040,074, 9,950,069 and 10,039,832
Copyright© 2025 UroGen Pharma, Inc.
All rights reserved.
ZUS-PI-001
PI-0017645/Ver. 1
| Patient Information ZUSDURI™ (zus-dur-ee) (mitomycin) for intravesical solution |
|
|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued: 06/2025 |
| What is ZUSDURI?
ZUSDURI is a prescription medicine used to treat adults with a type of cancer of the lining of the bladder called low-grade intermediate risk non-muscle invasive bladder cancer (LG-IR-NMIBC) after you have previously received bladder surgery to remove tumor and it did not work or is no longer working. It is not known if ZUSDURI is safe and effective for use in children. |
|
| Who should not receive ZUSDURI?
Do not receive ZUSDURI if you:
|
|
Before receiving ZUSDURI, tell your healthcare provider about all of your medical conditions, including if you:
|
|
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. | |
How will I receive ZUSDURI?
|
|
| What are the possible side effects of ZUSDURI? | |
| The most common side effects of ZUSDURI include: | |
|
|
| Call your doctor for medical advice about possible side effects. You may report side effects to FDA at 1-800-FDA-1088. You can also report side effects to UroGen Pharma at 1-855-987-6436. |
|
| General information about ZUSDURI.
Medicines are sometimes prescribed for purposes other than those listed in this Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about ZUSDURI that is written for healthcare professionals. |
|
| What are the ingredients of ZUSDURI?
Active ingredient: mitomycin Inactive ingredients: hydroxypropyl methylcellulose, mannitol, poloxamer, polyethylene glycol, and water for injection Distributed by: UroGen Pharma, Inc., Princeton, NJ 08540 ZUSDURI™ is a trademark and UroGen® is a registered trademark of UroGen Pharma, Ltd. U.S. Patent Nos. 9,040,074, 9,950,069 and 10,039,832 Copyright© 2025 UroGen Pharma, Inc. All rights reserved. For more information go to www.ZUSDURI.com or call 1-855-987-6436. |
|
ZUS-PPI-001
INSTRUCTIONS FOR PHARMACY (IFP) ZUSDURI™ (zus-dur-ee) (mitomycin) for intravesical solution
Purpose of this Instructions for Pharmacy
This Instructions for Pharmacy contains information on how to prepare ZUSDURI using pharmacy supplies and a Chilling Block or other means of chilling.
Intended Use of ZUSDURI
ZUSDURI (mitomycin) for intravesical solution is indicated for the treatment of adult patients with recurrent low-grade intermediate risk non-muscle invasive bladder cancer (LG-IR-NMIBC).
Important Information You Need to Know Before Reconstituting ZUSDURI
Reconstituted ZUSDURI must be prepared under chilled conditions. ZUSDURI cannot be prepared without a Chilling Block or other means of chilling.
Once reconstituted with sterile hydrogel, ZUSDURI will appear as a semisolid gel under room temperature conditions and as a viscous liquid under chilled conditions.
Preparation of ZUSDURI must be performed under aseptic conditions.
Storage Conditions and Handling
Before reconstitution, store ZUSDURI at room temperature, 20°C to 25°C (68°F to 77°F). Excursions are permitted between 15°C and 30°C (59°F and 86°F). Avoid excessive heat over 40°C (104°F). Protect from light.
Storage Instructions for Reconstituted ZUSDURI:
- Instill reconstituted ZUSDURI as soon as possible.
- If not used immediately, store reconstituted ZUSDURI:
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 7 days; or
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
- Discard 7 days after reconstitution.
- Protect from light.
- Avoid excessive heat over 40°C (104°F).
ZUSDURI is a hazardous drug. Procedures for Proper Handling and Disposal of hazardous drugs should be followed.
Supplies Needed
ZUSDURI available in a kit containing:
|  |
Pharmacy Supplies:
(Provided by your facility)
|
|
| Two 20 mL Luer lock syringes |  |
| Two 5 mL Luer lock syringes filled with 3 mL sterile water |  |
| Three OnGuard®2 CSTD vial adaptors |  |
| Four OnGuard®2 CSTD syringe adaptors |  |
| Chilling Block or other means of chilling (device design may vary) Note: The day before the preparation, place the Chilling Block in the freezer at -20°C to -12°C (-4°F to 10.4°F). Refer to the Chilling Block Instructions for Use for more information. | 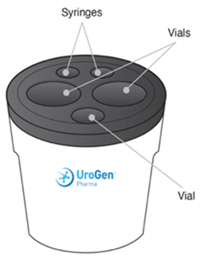 |
Steps to Prepare ZUSDURI Admixture
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frequently Asked Questions
| This Instructions for Pharmacy has been approved by the U.S. Food and Drug Administration. | |
| How do I connect the vial adaptor?
Place the vial adaptor over the vial and press down firmly. You will hear a snap which confirms successful attachment. | 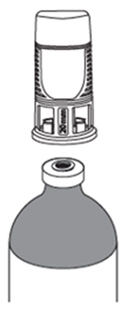 |
| How do I connect the syringe adaptor?
Screw the Luer lock end of the syringe adaptor onto the syringe hub until it is finger tight. | 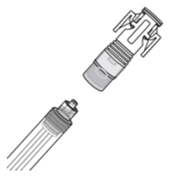 |
| How do I connect the syringe adaptor to the vial adaptor?
Place the syringe adaptor over the vial adaptor and align the tabs, then press down firmly. You will hear a snap which confirms successful attachment. | 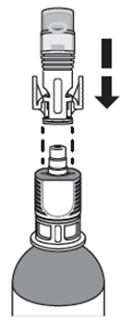 |
| How do I disconnect the syringe adaptor from the vial adaptor?
Pinch the tabs on the syringe adaptor to separate it from the vial adaptor. | 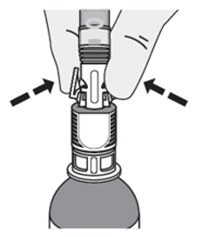 |
| Do I have to keep the vials upright when mixing? If so, why?
Yes, the vials should be kept upright when mixing to ensure the contents are fully mixed at the bottom of the vial. | 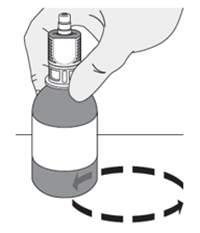 |
| I am having difficulty working with the drug product. It seems to be solidifying.
If you are having difficulty pushing or withdrawing the solution, place the components back in the Chilling Block or other means of chilling until it liquifies. |
|
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540
www.ZUSDURI.com
ZUSDURI™ is a trademark of UroGen Pharma, Ltd.
OnGuard® is a registered trademark of Simplivia Healthcare, Ltd.
Copyright© 2025 UroGen Pharma, Inc.
All rights reserved.
ZUS-IFP-001
IFP-0017643/Ver. 1
INSTRUCTIONS FOR ADMINISTRATION (IFA) ZUSDURI™ (zus-dur-ee) (mitomycin) for intravesical solution For Intravesical Instillation Only
|
| Read and follow this Instructions for Administration prior to each ZUSDURI instillation. |
Purpose of this Instructions for Administration
This Instructions for Administration contains information on how to instill ZUSDURI using the ZUSDURI vial and the devices listed under Supplies Needed, obtained by your facility.
Intended Use of ZUSDURI
ZUSDURI (mitomycin) for intravesical solution is indicated for the treatment of adult patients with recurrent low-grade intermediate risk non-muscle invasive bladder cancer (LG-IR-NMIBC).
Important Information You Need to Know Before Instilling ZUSDURI
ZUSDURI must be reconstituted by a healthcare professional prior to instillation. Reconstituted ZUSDURI will appear as a semisolid gel. Once chilled, ZUSDURI will convert to a viscous liquid for instillation.
Once instilled into the patient's bladder, ZUSDURI will convert to a semisolid gel, thereby exposing the lining of the bladder to mitomycin over a prolonged period of time.
Storage Instructions for Reconstituted ZUSDURI:
- Instill reconstituted ZUSDURI as soon as possible.
- If not used immediately, store reconstituted ZUSDURI:
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 7 days; or
- under refrigeration at 2℃ to 8℃ (36°F to 46°F) for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
- Discard 7 days after reconstitution.
- Protect from light.
- Avoid excessive heat over 40°C (104°F).
When ready to instill, chill ZUSDURI at -3°C to 5°C (27°F to 41°F) for about 20 minutes to revert it to a viscous liquid. See Steps A through C below for complete administration instructions.
ZUSDURI is a hazardous drug. Procedures for proper handling and disposal of hazardous drugs should be followed, as described below.
Supplies Needed
| One vial of reconstituted ZUSDURI, with resultant concentration of 80 mg per 60 mL.
(prepared and provided by your pharmacy) |  |
| Ice Bath Note: The ice bath should consist of ice and cold water and be large enough to submerge a ZUSDURI vial. |  |
| Plastic Bag |  |
Ancillary Supplies
When you administer ZUSDURI, you will need to use special adaptors for your catheter and syringes. These adaptors will create a closed pathway to protect you and your patient during and after administration. Fluid will only be able to pass between connection points when the adaptors are fully engaged, preventing drips or leaks.
Once the adaptors are connected, do not disconnect them. They are there for your protection.
| Urinary Catheter
One 12 Fr to 16 Fr with fixed Luer lock connector | 
|
| Catheter Adaptor
One OnGuard®2 CSTD Luer lock adaptor | 
|
| Administration Syringes
Four 20 mL polypropylene Luer lock syringes (BD Luer-Lok™ Syringe) | 
|
| Sterile Water Flush
One 5 mL Luer lock syringe filled with 3 mL sterile water |  |
| Syringe Adaptors
Five OnGuard®2 CSTD syringe adaptors | 
|
Instillation Instructions
- A.
-
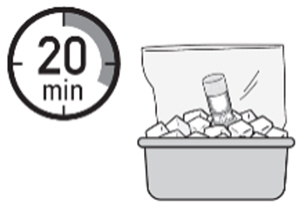
Chill the ZUSDURI
- 1.
- Place the ZUSDURI vial in a plastic bag.
- 2.
- Place the plastic bag in the ice bath for approximately 20 minutes.
Note: Ensure the ZUSDURI vial is completely immersed in the ice bath.
- B.
-
Prepare the Four Administration Syringes
- 1.
- Connect a syringe adaptor to the 3 mL sterile water syringe and set aside.
- 2.
- Connect syringe adaptors to the four 20 mL syringes. These will be the administration syringes.
Note: Once the adaptors are connected, do not disconnect them. They are there for your protection. - 3.
- Remove the ZUSDURI vial from the ice bath and verify the admixture is liquid. If the admixture is not liquid, place the vial bag back into the ice bath until the admixture liquefies.
- 4.
- Swirl the vial upright to ensure that ZUSDURI is uniformly mixed.
- 5.
- Slowly fill each of the four administration syringes with 14 mL of ZUSDURI admixture (for a total of 56 mL).
Note: there might be foaming in the syringe while withdrawing ZUSDURI. - 6.
- Place the four administration syringes in the plastic bag.
- 7.
- Place the plastic bag in the ice bath, ensuring the four administration syringes are completely immersed, for at least two minutes.
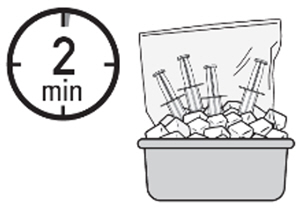
- C.
-
Instill ZUSDURI
 The contents of four administration syringes must be instilled in rapid succession without delay to avoid ZUSDURI solidification. Before proceeding:
The contents of four administration syringes must be instilled in rapid succession without delay to avoid ZUSDURI solidification. Before proceeding:- Ensure that all four administration syringes contain liquid ZUSDURI.
- Keep all four administration syringes chilled in the ice bath until instillation time.
- 1.
- Place the urinary catheter in the bladder and drain the urine.
- 2.
- Connect the catheter adaptor to the urinary catheter.
- 3.
- Connect an administration syringe to the urinary catheter.
- 4.
- Instill the ZUSDURI into the patient.
Note: Empty each administration syringe over approximately 30 seconds. ZUSDURI is viscous, even in a chilled state.
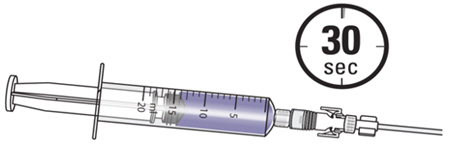
- 5.
- Disconnect the syringe adaptor from the catheter adaptor.
- 6.
- Immediately repeat these steps without delay for the remaining three administration syringes.
- 7.
- After all four administration syringes have been instilled, flush the urinary catheter with 3 mL of sterile water.
- 8.
- Wait 15 minutes before removing the urinary catheter from the patient.

- 9.
- Discard all ancillary supplies according to your facility's procedures for hazardous waste.
Frequently Asked Questions
| How do I connect the syringe adaptor?
Screw the Luer lock end of the syringe adaptor onto the syringe hub until it is finger tight. | 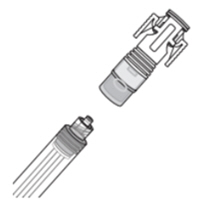 |
| How do I connect the syringe adaptor to the vial adaptor?
Place the syringe adaptor over the vial adaptor and align the tabs; then press down firmly. You will hear a snap which confirms successful attachment. | 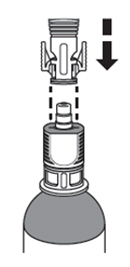 |
| How do I disconnect the syringe adaptor from the vial adaptor?
Pinch the tabs on the syringe adaptor to separate it from the vial adaptor. | 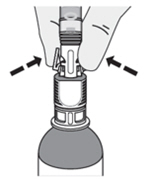 |
| I am having a hard time working with ZUSDURI. It seems to be solidifying.
If you are having difficulty pushing or withdrawing ZUSDURI, recap and place the components back into the ice bath until ZUSDURI liquifies. The vial may be placed upside down within the bag in the ice bath. | 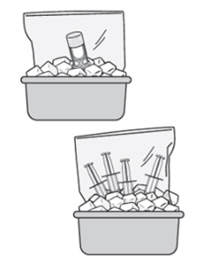 |
| How do I connect the catheter adaptor to the urinary catheter?
Screw the Luer lock end of the catheter adaptor onto the urinary catheter connector until it is finger tight. | 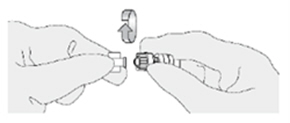 |
| How do I connect the syringe adaptor to the catheter adaptor?
Place the syringe adaptor in front of the catheter adaptor and push it on firmly. You will hear a snap which confirms successful attachment. | 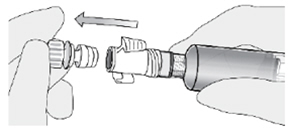 |
| How do I disconnect the syringe adaptor from the catheter adaptor?
While holding the urinary catheter steady and fixed in its position, pinch the tabs on the syringe adaptor to separate it from the catheter adaptor. | 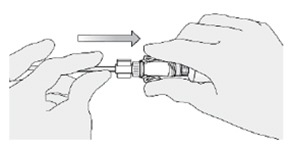 |
This "Instructions for Administration" has been approved by the U.S. Food and Drug Administration.
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540
www.ZUSDURI.com
ZUSDURI™ is a trademark of UroGen Pharma, Ltd.
OnGuard® is a registered trademark of Simplivia Healthcare Ltd.
BD Luer-Lok™ is a trademark of Becton, Dickinson and Company.
Copyright© 2025 UroGen Pharma, Inc.
All rights reserved.
ZUS-IFA-001
IFA-0017644/Ver. 1
PRINCIPAL DISPLAY PANEL - 40 mg Kit Carton
NDC 72493-106-03
Zusduri™
(mitomycin) for intravesical solution
40 mg per Vial
Attention Pharmacist: Reconstitution
is required prior to dispensing.
See the Instructions for Pharmacy
before proceeding.
SINGLE-DOSE KIT
Warning: For Intravesical Use Only
Rx Only
Contents of Kit:
- 2 Single-Dose Vials of ZUSDURI (mitomycin) for intravesical solution, 40 mg per Vial.
- 1 Single-Dose Vial of Sterile Hydrogel, 60 mL per Vial
- 1 Admixture Label
- Full Prescribing Information
- Instructions for Pharmacy
- Instructions for Administration
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F).
Avoid excessive heat over 40°C (104°F). Protect from light.
Store reconstituted ZUSDURI at 2°C to 8°C (36°F to 46°F) for up to 7 days.
Alternatively, reconstituted ZUSDURI may be stored under refrigeration at 2°C to 8°C (36°F to 46°F)
for up to 6 days followed by no more than 24 hours at room temperature, 20°C to 25°C (68°F to 77°F).
Avoid excessive heat over 40°C (104°F).
Protect reconstituted ZUSDURI from light.
When ready to instill, chill ZUSDURI at -3°C to 5°C (27°F to 41°F) for about 20 minutes to revert it to a
viscous liquid.
Recommended Dosage: See Full Prescribing Information.
Warning: Hazardous Drug
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540
UroGen®
Pharma

PRINCIPAL DISPLAY PANEL - 40 mg Vial Label
NDC 72493-104-40
Single-Dose Vial
Sterile
Zusduri™
(mitomycin) for intravesical solution
40 mg per Vial
See Instructions for Pharmacy for preparation instructions
Must be Reconstituted with Sterile Hydrogel Before Use
Warning: For Intravesical Use Only
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C
(59°F and 86°F). Avoid excessive heat over 40°C (104°F). Protect from light.
Each vial contains: Mitomycin 40 mg and mannitol 80 mg.
Recommended Dosage: See Full Prescribing Information.
Distributed by:
UroGen Pharma, Inc.
Princeton, NJ 08540
Lot
Exp
Warning: Hazardous Drug
Rx Only
UroGen®
Pharma
620565
LBL-0017642/Ver. 1

PRINCIPAL DISPLAY PANEL - 60 mL Vial Label
NDC 72493-105-60
Single-Dose Vial
Sterile Hydrogel
For use in preparation of ZUSDURI™
(mitomycin) for intravesical solution
Not for Direct Administration
See Instructions for Pharmacy for
preparation instructions
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted between 15°C and 30°C (59°F and 86°F).
Avoid excessive heat over 40°C (104°F).
Rx Only
60 mL per Vial
LBL-0017641/Ver. 1

| ZUSDURI
mitomycin kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - UroGen Pharma, Inc (081130487) |
| Registrant - UroGen Pharma, Ltd. (534155213) |
More about Zusduri (mitomycin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Latest FDA alerts (1)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antibiotics/antineoplastics