L-Methyl-MC Tablets: Package Insert / Prescribing Info
Package insert / product label
Generic name: levomefolate calcium, riboflavin, cyanocobalamin, pyridoxine
Dosage form: tablet, coated
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
L-Methyl-MC Tablets Description
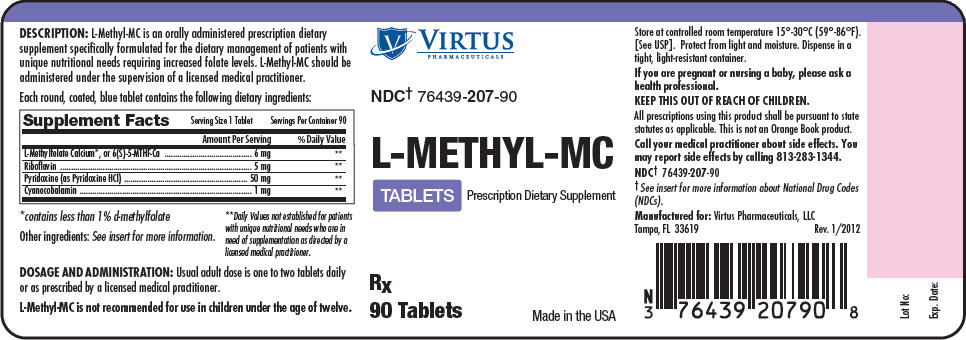
L-Methyl-MC is an orally administered prescription dietary supplement specifically formulated for the dietary management of patients with unique nutritional needs requiring increased folate levels.
L-Methyl-MC should be administered under the supervision of a licensed medical practitioner. Each round, coated, blue tablet contains the following dietary ingredients:
| Serving Size: 1 tablet | Servings Per Container: 90 | |
|---|---|---|
| Amount Per Serving | % Daily Value | |
| L-Methylfolate Calcium*, or 6(S)-5-MTHF-Ca | 6 mg | † |
| Riboflavin | 5 mg | † |
| Pyridoxine (as Pyridoxine HCl) | 50 mg | † |
| Cyanocobalamin | 1 mg | † |
Other Ingredients: Croscarmellose Sodium, Dicalcium Phosphate, Magnesium Stearate, Microcrystalline Cellulose, Silicon Dioxide, Stearic Acid, and Film Coat (FD&C Blue #2 Lake, Hydroxypropyl Cellulose, Hypromellose, Polyethylene Glycol, Propylene Glycol, and Titanium Dioxide).
FOLATE REGULATION
The term "folate" are B vitamins that include folic acid and any forms of active pteroylglutamates regardless of the reduction state of the molecule. Folates, or vitamin B9, are primarily hydrolyzed in the intestinal jejunum and the liver to the active circulating form of folate, l-methylfolate, with an intermediate stable form, 5,10-methylenetetrahydrofolate.
Individuals with genetic polymorphisms for the genes coding methylenetetrahydrofolate reductase (MTHFR) may not be capable of utilizing or metabolizing folic acid adequately for the vitamin B12 dependent methylation cycle.
Folic acid, including reduced forms1 such as folinic acid, may obscure pernicious anemia above 0.1 mg doses, and must be administered under the supervision of a licensed medical practitioner.
The 1971, 1972, 1973, 1980, 1984, 2000, and 2010 Federal Register Notices addressed this concern while establishing that increased folate was proper therapy in megaloblastic anemias - specifically where homocysteine levels were elevated or risk of neural tube defects (NTDs) was at issue. The Federal Register Notice of August 2, 1973 (38 FR 20750) specifically states that:
Dietary supplement preparations are available without a prescription (21 CFR 121.1134). Levels higher than dietary supplement amounts are available only with a prescription.
Folic acid - including reduced forms, may be added to medical foods undefined in section 5(b)(3) of the Orphan Drug Act (21 USC 360ee(b)(3)), or to food (21 CFR 172.345).
- 1
- It is not known whether or not l-methylfolate can obscure pernicious anemia above 0.1 mg doses, so caution is advised also with this form of folate.
Indications and Usage for L-Methyl-MC Tablets
L-Methyl-MC is indicated for the distinct nutritional requirements of patients in need of dietary supplementation as determined by a licensed medical practitioner.
L-Methyl-MC should be administered under the supervision of a licensed medical practitioner.
Contraindications
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Precautions
General
Folate, when administered as a single agent in doses about 0.1 mg daily, may obscure the detection of vitamin B12 deficiency (specifically, the administration of folic acid may reverse the hematological manifestations of B12 deficiency, including pernicious anemia, while not addressing the neurological manifestations). Folate therapy alone is inadequate for treatment of a vitamin B12 deficiency.
PATIENT INFORMATION
L-Methyl-MC is a prescription dietary supplement to be used only under licensed medical supervision.
DRUG INTERACTIONS
Drugs which may interact with folate include:
- Antiepileptic drugs (AED): The AED class including, but not limited to, phenytoin, carbamazepine, primidone, valproic acid, fosphenytoin, valproate, phenobarbital and lamotrigine have been shown to impair folate absorption and increase the metabolism of circulating folate.
- Additionally, concurrent use of folic acid has been associated with enhanced phenytoin metabolism, lowering the level of the AED in the blood and allowing breakthrough seizures to occur. Caution should be used when prescribing this product among patients who are receiving treatment with phenytoin and other anticonvulsants.
- Capecitabine: Folinic acid (5-formyltetrahydrofolate) may increase the toxicity of Capecitabine.
- Cholestyramine: Reduces folic acid absorption and reduces serum folate levels.
- Colestipol: Reduces folic acid absorption and reduces serum folate levels.
- Cycloserine: Reduces folic acid absorption and reduces serum folate levels.
- Dihydrofolate Reductase Inhibitors (DHFRI): DHFRIs block the conversion of folic acid to its active forms, and lower plasma and red blood cell folate levels. DHFRIs include aminopterin, methotrexate, pyrimethamine, triamterene, and trimethoprim.
- Fluoxetine: Fluoxetine exerts a noncompetitive inhibition of the 5-methyltetrahydrofolate active transport in the intestine.
- Isotretinoin: Reduced folate levels have occurred in some patients taking isotretinoin.
- L-dopa, triamterene, colchicine, and trimethoprim may decrease plasma folate levels.
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDs have been shown to inhibit some folate dependent enzymes in laboratory experiments. NSAIDs include ibuprofen, naproxen, indomethacin and sulindac.
- Oral Contraceptives: Serum folate levels may be depressed by oral contraceptive therapy.
- Methylprednisolone: Reduced serum folate levels have been noted after treatment with methylprednisolone.
- Pancreatic Enzymes: Reduced serum folate levels have occurred in some patients taking pancreatic extracts, such a pancreatin and pancrelipase.
- Pentamidine: Reduced folate levels have been seen with prolonged intravenous pentamidine.
- Pyrimethamine: High levels of folic acid may result in decreased serum levels of pyrimethamine.
- Smoking and Alcohol: Reduced serum folate levels have been noted.
- Sulfasalazine: Inhibits the absorption and metabolism of folic acid.
- Metformin treatment in patients with type 2 diabetes decreases serum folate.
- Warfarin can produce significant impairment in folate status after a 6-month therapy.
- Folinic acid may enhance the toxicity of fluorouracil.
- Concurrent administration of chloramphenicol and folinic acid in folate-deficient patients may result in antagonism of the haematopoietic response to folate.
- Caution should be exercised with the concomitant use of folinic acid and trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection as it is associated with increased rates of treatment failure and mortality in a placebo controlled study.
Drugs which interact with vitamin B6:
- Vitamin B6 should not be given to patients receiving the drug levodopa because the action of levodopa is antagonized by vitamin B6. However, vitamin B6 may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
Drugs which may interact with vitamin B12:
- Antibiotics, cholestyramine, colchicines, colestipol, metformin, para-aminosalicylic, and potassium chloride may decrease the absorption of vitamin B12.
- Nitrous oxide can produce a functional vitamin B12 deficiency.
Adverse Reactions/Side Effects
Allergic sensitization has been reported following both oral and parental administration of folic acid, and may possibly occur with other forms of folate.
Paresthesia, somnolence, nausea, and headaches have been reported with vitamin B6.
Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body have been associated with vitamin B12.
If headaches occur with the use of this product, consult your medical practitioner.
Related/similar drugs
L-Methyl-MC Tablets Dosage and Administration
Usual adult dose is one to two tablets daily or as prescribed by a licensed medical practitioner. L-Methyl-MC is not recommended for use in children under the age of twelve.
How is L-Methyl-MC Tablets supplied
L-Methyl-MC is supplied as round, coated, blue tablets debossed "BP" on top and "850" on bottom, dispensed in bottles of 90 tablets.
NDC2 : 76439-207-90
- 2
- This product is a dietary supplement that due to increased folate levels (AUG 2, 1973 38 FR 20750), requires an Rx on the label because of increased risk associated with masking of B12 deficiency. As such, this product requires licensed medical supervision, an Rx status, and a National Drug Code (NDC) as required by pedigree reporting requirements.
STORAGE
Store at Controlled Room Temperature 15°-30°C (59°-86°F). [See USP]. Protect from light and moisture. Dispense in a tight, light-resistant container.
Call your medical practitioner about side effects. You may report side effects by calling (813)-283-1344.
KEEP THIS OUT OF THE REACH OF CHILDREN.
| L-METHYL MC
levomefolate calcium, riboflavin, cyanocobalamin, and pyridoxine tablet, coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Virtus Pharmaceuticals (969483143) |
Frequently asked questions
More about L-Methyl-MC (multivitamin)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
Professional resources
Other brands
MVI Adult, Nephplex Rx, Folplex, Nephrocaps, ... +12 more