Hypertonic Dextrose Injection: Package Insert / Prescribing Info
Package insert / product label
Generic name: dextrose
Dosage form: injection
Drug class: Glucose elevating agents
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
Hypertonic Dextrose Injection Description
| Composition Each 100 mL contains: | |||||
|---|---|---|---|---|---|
| Solution | Hydrous Dextrose USP | Calculated Osmolarity mOsmol/liter | Calories per liter | pH | Calc. Specific Gravity |
| Water for Injection USP qs | |||||
| 20% Dextrose Injection, USP | 20 g | 1010 | 680 | 4.6 (3.2–6.5) | 1.07 |
| 30% Dextrose Injection, USP | 30 g | 1515 | 1020 | 4.6 (3.2–6.5) | 1.10 |
| 40% Dextrose Injection, USP | 40 g | 2020 | 1360 | 4.6 (3.2–6.5) | 1.14 |
| 50% Dextrose Injection, USP | 50 g | 2525 | 1700 | 4.6 (3.2–6.5) | 1.17 |
| 60% Dextrose Injection, USP | 60 g | 3030 | 2040 | 4.6 (3.2–6.5) | 1.20 |
| 70% Dextrose Injection, USP | 70 g | 3530 | 2380 | 4.6 (3.2–6.5) | 1.23 |
Hypertonic Dextrose Injections, USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents.
The formula of the active ingredient is:
| Ingredient | Molecular Formula | Molecular Weight |
|---|---|---|
| Hydrous Dextrose USP | 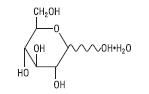 | 198.17 |
Hypertonic Dextrose Injection - Clinical Pharmacology
Hypertonic Dextrose Injections, USP provide calories and are a source of water for hydration. These solutions are capable of inducing diuresis depending on the clinical condition of the patient.
Dextrose is readily metabolized, may decrease losses of body protein and nitrogen, promotes glycogen deposition and decreases or prevents ketosis if sufficient doses are provided.
Hypertonic dextrose solutions provide a maximum source of calories in a minimal fluid volume.
Indications and Usage for Hypertonic Dextrose Injection
These intravenous solutions are indicated for use in adults and pediatric patients as sources of calories and water for hydration.
Contraindications
Solutions containing dextrose may be contraindicated in patients with hypersensitivity to corn products.
Administration of solutions containing hypertonic dextrose is contraindicated in patients with intracranial or intraspinal hemorrhage, diabetic coma or delirium tremens, especially if such patients are already dehydrated.
Warnings
The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentration.
WARNING: These products contain aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Solutions containing dextrose without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility of agglomeration.
Excessive administration of potassium-free dextrose solutions may result in significant hypokalemia. Serum potassium levels should be maintained and potassium supplemented as required.
Hypertonic dextrose solutions (above approximately 600 mOsmol/liter) may cause thrombosis if infused via a peripheral vein. It is, therefore, advisable to administer such solutions via an intravenous catheter placed in a large central vein, preferably the superior vena cava. Concentrations of Dextrose Injection, USP 20% and greater should be administered exclusively by this route.
Rapid administration of solutions containing hypertonic dextrose may produce significant hyperglycemia and hyperosmolar syndrome. Patients should be observed for signs of mental confusion and loss of consciousness, especially in patients with chronic uremia or carbohydrate intolerance. If undetected and untreated, this can lead to osmotic dehydration, hyperosmolar coma and death.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
Precautions
General
These solutions should be used with care in patients with hypervolemia, renal insufficiency, urinary tract obstruction, or impending or frank cardiac decompensation.
Solutions containing dextrose should be used with caution in patients with overt or known subclinical diabetes mellitus or carbohydrate intolerance for any reason.
Prolonged administration of solutions containing hypertonic dextrose may adversely affect the production of insulin in some patients. To avoid this potential danger, and to minimize hyperglycemia and consequent glycosuria, it may be necessary to add insulin to the infusion. Periodically measure blood and urinary glucose.
Essential electrolytes, minerals, and vitamins should be supplied as needed.
When infusions of concentrated dextrose are discontinued, it is advisable to substitute a solution of 5% or 10% dextrose to prevent rebound hypoglycemia.
Hypokalemia may develop during parenteral administration of hypertonic dextrose solutions. Sufficient amounts of potassium should be added to dextrose solutions administered to fasting patients with good renal function, especially those on digitalis therapy.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result.
These solutions are intended for intravenous administration using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours.
Do not use unless solution is clear, closure is intact and vacuum is present.
These drug products contain no more than 25 µg/L of aluminum.
Laboratory Tests
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation. Significant deviations from normal concentrations may require tailoring of the electrolyte pattern, in these or alternative solutions.
Drug Interactions
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with Hypertonic Dextrose Injections, USP have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Hypertonic Dextrose Injections, USP. It is also not known whether Hypertonic Dextrose Injections, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hypertonic Dextrose Injections, USP should be given to a pregnant woman only if clearly needed.
Labor and Delivery
As reported in the literature, dextrose solutions have been administered during labor and delivery. Caution should be exercised, and the fluid balance, glucose and electrolyte concentrations and acid-base balance, of both mother and fetus should be evaluated periodically or whenever warranted by the condition of the patient or fetus.
Nursing Mothers
Because many drugs are excreted in human milk, caution should be exercised when Hypertonic Dextrose Injections, USP are administered to nursing women.
Pediatric Use
In neonates or in very small infants even small volumes of fluid may affect fluid and electrolyte balance. Care must be exercised in treatment of neonates, especially pre-term neonates, whose renal function may be immature and whose ability to excrete fluid and solute loads may be limited. Fluid intake, urine output, and serum electrolytes should be monitored closely.
Serum glucose concentrations should be frequently monitored when dextrose is prescribed to pediatric patients, particularly infants, neonates, and low birth weight infants.
See WARNINGS and DOSAGE AND ADMINISTRATION.
Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
These drugs are known to be substantially excreted by the kidney, and the risk of toxic reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
See WARNINGS.
Adverse Reactions/Side Effects
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
Too rapid infusion of hypertonic solutions may cause local pain and venous irritation. Rate of administration should be adjusted according to tolerance. Use of the largest peripheral vein and a small bore needle is recommended. (See DOSAGE AND ADMINISTRATION.)
Dextrose may be administered at a rate of 0.5 g/kg/hour without producing glycosuria. Hyperglycemia and glycosuria may be a function of rate of administration or metabolic insufficiency. Appropriate therapy may include slowing of the infusion rate and administration of insulin.
Too rapid administration of hypertonic dextrose solutions may result in hyperosmolar syndrome with signs of mental confusion and/or loss of consciousness. If undetected and untreated, this can lead to osmotic dehydration, hyperosmolar coma and death.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
Related/similar drugs
Overdosage
In the event of a fluid or solute overload during parenteral therapy, reevaluate the patient's condition and institute appropriate corrective treatment.
Hypertonic Dextrose Injection Dosage and Administration
These solutions are for intravenous use only. Hypertonic, administer only after dilution via central venous catheter.
Dosage is to be directed by a physician and is dependent upon age, weight, clinical condition of the patient and laboratory determinations. Frequent laboratory determinations and clinical evaluation are essential to monitor changes in blood glucose and electrolyte concentrations, and fluid and electrolyte balance during prolonged parenteral therapy.
Fluid administration should be based on calculated maintenance or replacement fluid requirements for each patient.
Dextrose may be administered to normal individuals at a rate of 0.5 g/kg/hour without producing glycosuria. At the maximum infusion rate of 0.8 g/kg/hour, approximately 95% of the dextrose is retained.
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Pediatric Use
There is no specific pediatric dose. The dose is dependent on weight, clinical condition, and laboratory results. Follow recommendations of appropriate pediatric reference text. (See WARNINGS and PRECAUTIONS.)
How is Hypertonic Dextrose Injection supplied
Hypertonic Dextrose Injections, USP are supplied sterile and nonpyrogenic in full fill and partial fill glass containers with solid stoppers. The 1000 mL containers are packaged 6 per case and the 500 mL containers are packaged 12 per case.
| Container | ||||
|---|---|---|---|---|
| NDC | Canada DIN | Cat. No. | Size | Fill |
| 20% Dextrose Injection, USP | ||||
| 0264-1250-55 | (Canada DIN 01924257) | S1250-50SS | 1000 mL | 500 mL |
| 0264-1251-55 | S1251-SS | 500 mL | 500 mL | |
| 30% Dextrose Injection, USP | ||||
| 0264-1240-55 | S1240-50SS | 1000 mL | 500 mL | |
| 40% Dextrose Injection, USP | ||||
| 0264-1260-55 | (Canada DIN 01947230) | S1260-10SS | 1000 mL | 500 mL |
| 50% Dextrose Injection, USP | ||||
| 0264-1280-55 | (Canada DIN 01924273) | S1280-SS | 1000 mL | 1000 mL |
| 0264-1280-50 | S1280-50SS | 1000 mL | 500 mL | |
| 0264-1281-55 | S1281-SS | 500 mL | 500 mL | |
| 60% Dextrose Injection, USP | ||||
| 0264-1270-50 | S1270-50SS | 1000 mL | 500 mL | |
| 70% Dextrose Injection, USP | ||||
| 0264-1290-55 | S1290-SS | 1000 mL | 1000 mL | |
| 0264-1290-50 | (Canada DIN 01924265) | S1290-50SS | 1000 mL | 500 mL |
Directions for Use of B. Braun Glass Containers with Solid Stoppers
Designed for use with a vented set. Use 18 to 22 gauge needle size for spiking/admixing or withdrawing solutions from the glass bottle.
Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter; check the bottle for cracks or other damage. In checking for cracks, do not be confused by normal surface marks and seams on the bottom and sides of the bottle. These are not flaws. Look for bright reflections that have depth and penetrate into the wall of the bottle. Reject any such bottle.
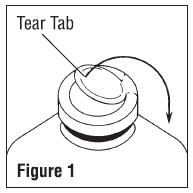
- To remove the outer closure, lift the tear tab and pull up, over, and down until it is below the stopper (see Figure 1). Use a circular pulling motion on the tab until it breaks away.
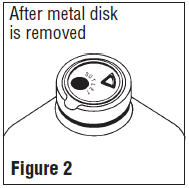
- Grasp and remove the metal disk, exercising caution not to touch the exposed sterile stopper surface.
Warning: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
- Refer to Directions for Use of the set being used. Insert the set spike into the large round outlet port of the stopper and hang container.
- After admixture and during administration, reinspect the solution frequently. If any evidence of solution contamination or instability is found or if the patient exhibits any signs of fever, chills or other reactions not readily explainable, discontinue administration immediately and notify the physician.
- When adding medication to the container during administration, swab the triangular medication site, inject medication and mix thoroughly by gentle agitation.
- Spiking, additions, or transfers should be made immediately after exposing the sterile stopper surface. Check for vacuum at first puncture of stopper. Admixture by needle or syringe should be made through the triangular ( ∇ ) medication site; contents should be drawn by vacuum into the bottle. Admixture by spiked vial should be through the outlet port (see Figure 2). If contents of initial addition are not drawn into the bottle, vacuum is not present and the unit should be discarded. Each addition/transfer will reduce the vacuum remaining in the bottle.
- If the first puncture of the stopper is the administration set spike, insert the spike fully into the outlet port of the stopper and promptly invert the bottle. Verify vacuum by observing rising air bubbles. Do not use the bottle if vacuum is not present.
- If admixture or set insertion is not performed immediately following removal of protective metal disk, swab stopper surface.
Caution: Large partial fill containers (one liter and larger) have high vacuum to facilitate large additions or transfers. This creates an implosion hazard and requires special care in use and handling.
Notice: Variation in color between different lots, and between units of the same lot, is normal for carbohydrate solutions, and electrolyte solutions containing sugars. These color differences have no effect on the therapeutic value of the solutions.
Distinction should be made between color (tint) and clarity (transparency). These solutions may be colored and are satisfactory for use if solution is clear and vacuum is present.
B. Braun Medical Inc.
Irvine, CA 92614-5895 USA
Made in USA
In Canada, distributed by:
B. Braun Medical Inc.
Scarborough, Ontario M1H 2W4
Y36-002-674
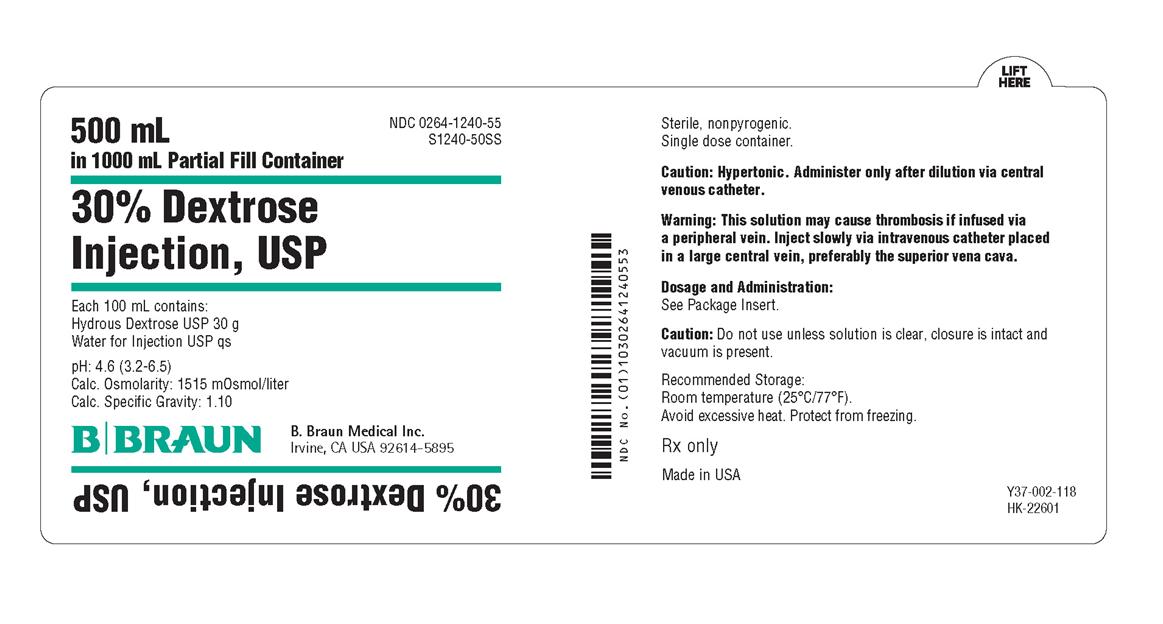
PRINCIPAL DISPLAY PANEL - 500 mL Container Label
500 mL
in 1000 mL Partial Fill Container
NDC 0264-1240-55
S1240-50SS
30% Dextrose
Injection, USP
Each 100 mL contains:
Hydrous Dextrose USP 30 g
Water for Injection USP qs
pH 4.6 (3.2-6.5)
Calc. Osmolarity: 1515 mOsmol/liter
Calc. Specific Gravity: 1.10
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
Sterile, nonpyrogenic.
Single dose container.
Caution: Hypertonic. Administer only after dilution via central
venous catheter.
Warning: This solution may cause thrombosis if infused via
a peripheral vein. Inject slowly via intravenous catheter placed
in a large central vein, preferably the superior vena cava.
Dosage and Administration:
See Package Insert.
Caution: Do not use unless solution is clear, closure is intact and
vacuum is present.
Recommended Storage:
Room temperature (25°C/77°F).
Avoid excessive heat. Protect from freezing.
Rx only
Made in USA
Y37-002-118
HK-22601
PRINCIPAL DISPLAY PANEL - 500 mL Container Label
500 mL
in 1000 mL Partial Fill Container
NDC 0264-1280-50
S1280-50SS
50%
DEXTROSE
INJECTION, USP
Each 100 mL contains:
Hydrous Dextrose USP 50 g
Water for Injection USP qs
pH: 4.6 (3.2-6.5)
Calc. Osmolarity: 2525 mOsmol/liter
Calc. Specific Gravity: 1.17
B. Braun Medical Inc.
Irvine, CA 92614-5895 USA
Sterile, nonpyrogenic. Single dose container.
Caution: Hypertonic. Administer only after dilution via
central venous catheter.
Warning: This solution may cause thrombosis if infused via a
peripheral vein. Inject slowly via intravenous catheter placed
in a large central vein, preferably the superior vena cava.
Dosage and Administration:
See Package Insert.
Caution: Do not use unless solution is clear, closure is intact
and vacuum is present.
Recommended Storage:
Room temperature (25°C/77°F). Avoid excessive heat.
Protect from freezing.
Rx only
Made in USA
HK-22602
Y37-002-261 LD-290-1

PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
1000 mL
Canada DIN 01924273
NDC 0264-1280-55
S1280-SS
50%
DEXTROSE
INJECTION, USP
NOT FOR DIRECT INFUSION MUST BE DILUTED
Each 100 mL contains:
Hydrous Dextrose USP 50 g
Water for Injection USP qs
pH: 4.6 (3.2-6.5), Hypertonic
Calc. Osmolarity: 2525 mOsmol/liter
Calc. Specific Gravity: 1.17
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
Sterile, nonpyrogenic.
Single dose container.
Dosage and Administration:
See Package Insert.
Caution: Do not use unless solution is clear, closure is intact
and vacuum is present.
Recommended Storage:
Room temperature (25°C/77°F).
Avoid excessive heat. Protect from freezing.
Rx only
In Canada, distributed by:
B. Braun Medical Inc.
Scarborough, Ontario M1H 2W4
Made in USA
Y37-002-124
HK-22602

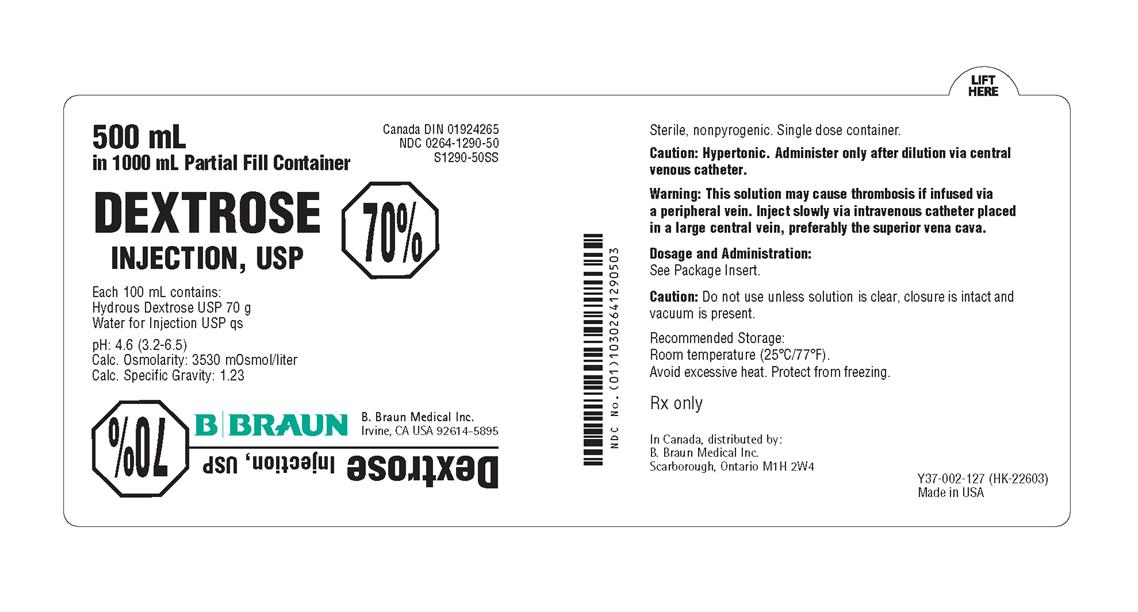
PRINCIPAL DISPLAY PANEL - 500 mL Container Label
500 mL
in 1000 mL Partial Fill Container
Canada DIN 01924265
NDC 0264-1290-50
S1290-50SS
DEXTROSE
INJECTION, USP
70%
Each 100 mL contains:
Hydrous Dextrose USP 70 g
Water for Injection USP qs
pH 4.6 (3.2-6.5)
Calc. Osmolarity: 3530 mOsmol/liter
Calc. Specific Gravity: 1.23
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
Sterile, nonpyrogenic. Single dose container.
Caution: Hypertonic. Administer only after dilution via central
venous catheter.
Warning: This solution may cause thrombosis if infused via
a peripheral vein. Inject slowly via intravenous catheter placed
in a large central vein, preferably the superior vena cava.
Dosage and Administration:
See Package Insert.
Caution: Do not use unless solution is clear, closure is intact and
vacuum is present.
Recommended Storage:
Room temperature (25°C/77°F).
Avoid excessive heat. Protect from freezing.
Rx only
In Canada, distributed by:
B. Braun Medical Inc.
Scarborough, Ontario M1H 2W4
Y37-002-127 (HK-22603)
Made in USA
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
1000 mL
NDC 0264-1290-55
S1290-SS
DEXTROSE INJECTION, USP
70%
NOT FOR DIRECT INFUSION
MUST BE DILUTED
Each 100 mL contains:
Hydrous Dextrose USP 70 g
Water for Injection USP qs
pH: 4.6 (3.2-6.5), Hypertonic
Calc. Osmolarity: 3530 mOsmol/liter
Calc. Specific Gravity: 1.23
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
Sterile, nonpyrogenic.
Single dose container.
Dosage and Administration:
See Package Insert.
Caution: Do not use unless solution is clear, closure is intact
and vacuum is present.
Recommended Storage:
Room temperature (25°C/77°F). Avoid excessive heat.
Protect from freezing.
Rx only
Made in USA
Y37-002-128
HK-22603

| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HYPERTONIC DEXTROSE
dextrose solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - B. Braun Medical Inc. (002397347) |
Frequently asked questions
- How long does metformin take to work?
- Is psyllium an affordable weight loss option to Ozempic?
- A1C levels: Dangerous vs normal and how to lower It?
More about glucose
- Check interactions
- Compare alternatives
- Reviews (3)
- Drug images
- Side effects
- Support group
- Drug class: glucose elevating agents
Patient resources
Professional resources
- Dextrose 50% Injection prescribing information
- Dextrose Injection (FDA)
- Dextrose Injection 10% (FDA)
- Dextrose Injection 70% (FDA)
- Dextrose Injection Concentrated (FDA)

