Floriva Plus: Package Insert / Prescribing Info
Package insert / product label
Generic name: fluoride, multivitamin
Dosage form: oral drops
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
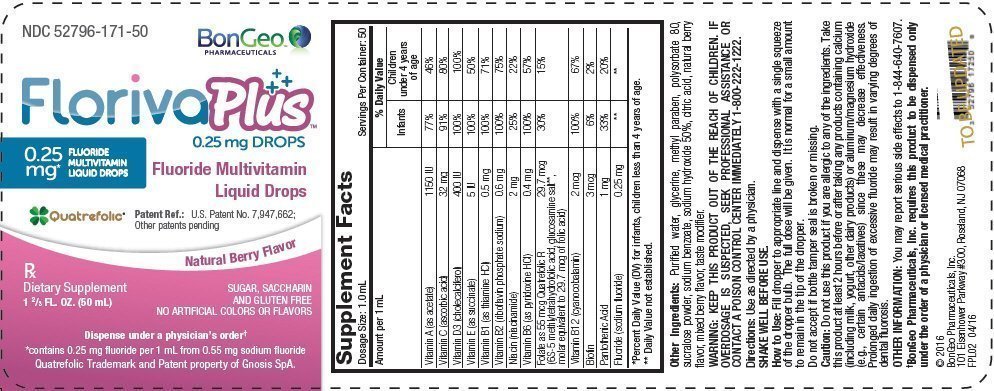
| Supplement Facts | |||
|---|---|---|---|
| Dosage Size: 1.0 mL | Servings Per Container: 50 | ||
| Amount per 1 mL | % Daily Value | ||
| Infants | Children under 4 years of age | ||
| *Percent Daily Value (DV) for infants, children less than 4 years of age. | |||
|
|||
| Vitamin A (as acetate) | 1150 IU | 77% | 46% |
| Vitamin C (ascorbic acid) | 32 mg | 91% | 80% |
| Vitamin D3 (cholecalciferol) | 400 IU | 100% | 100% |
| Vitamin E (as succinate) | 5 IU | 100% | 50% |
| Vitamin B1 (as thiamine HCl) | 0.5 mg | 100% | 71% |
| Vitamin B2 (riboflavin phosphate sodium) | 0.6 mg | 100% | 75% |
| Niacin (niacinamide) | 2 mg | 25% | 22% |
| Vitamin B6 (as pyridoxine HCl) | 0.4 mg | 100% | 57% |
| Folate as 55 mcg Quatrefolic R | 29.7 mcg | 30% | 15% |
| (6S-5 methlytetrahydrofolic acid, glucosamine salt*, molar equivalent to 29.7 mcg of folic acid) | |||
| Vitamin B12 (cyanocobalamin) | 2 mcg | 100% | 67% |
| Biotin | 3 mcg | 6% | 2% |
| Pantothenic Acid | 1 mg | 33% | 20% |
| Fluoride (sodium fluoride) | 0.25 mg | * | * |
Other Ingredients: Purified water, glycerine, methyl paraben, polysorbate 80, sucralose powder, sodium benzoate, sodium hydroxide 50%, citric acid, natural berry flavor, mixed berry flavor, taste modifier.
Warnings
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. IF OVERDOSAGE IS SUSPECTED, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY 1-800-222-1222.
Floriva Plus Dosage and Administration
Fill dropper to appropriate line and dispense with a single squeeze of the dropper bulb. The full dose will be given. It is normal for a small amount to remain in the tip of the dropper.
Do not accept if bottle tamper seal is broken or missing.
Related/similar drugs
Precautions
Do not use this product if you are allergic to any of the ingredients. Take this product at least 2 hours before or after taking any products containing calcium (including milk, yogurt, other dairy products) or aluminum/magnesium hydroxide (e.g., certain antacids/laxatives) since these may decrease effectiveness. Prolonged daily ingestion of excessive fluoride may result in varying degrees of dental fluorosis.
Storage and Handling
You may report serious side effects to 1-844-640-7607.
†BonGeo Pharmaceuticals, Inc. requires this product to be dispensed only under the order of a physician or licensed medical practitioner.
PRINCIPAL DISPLAY PANEL - 50 mL Container Label
NDC 52796-171-50
BonGeo™
PHARMACEUTICALS
FlorivaPlus™
0.25 mg DROPS
0.25
mg*
FLUORIDE
MULTIVITAMIN
LIQUID DROPS
Fluoride Multivitamin
Liquid Drops
Quatrefolic®
Patent Ref.: U.S. Patent No. 7,947,662;
Other patents pending
Natural Berry Flavor
Rx
Dietary Supplement
1 ⅔ FL. OZ. (50 mL)
SUGAR, SACCHARIN
AND GLUTEN FREE
NO ARTIFICIAL COLORS OR FLAVORS
Dispense under a physician's order†
*contains 0.25 mg fluoride per 1 mL from 0.55 mg sodium fluoride
Quatrefolic Trademark and Patent property of Gnosis SpA.
| FLORIVA PLUS
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, biotin, pantothenic acid, and sodium fluoride solution/ drops |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BonGeo Pharmaceuticals, Inc. (964822022) |
More about multivitamin with fluoride
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
- En español
Patient resources
- Multivitamins with fluoride drug information
- Pediatric Multivitamin Chewables with Fluoride
- Pediatric Multivitamin Drops with Fluoride
Professional resources
- Multi Vitamin Fluoride Drops prescribing information
- Multi Vitamin with Fluoride (FDA)
- Multi-Vit with Fluoride Drops (FDA)
- MultiVit with Fluoride Chewable Tablets (FDA)
- Multivitamin with Fluoride Chewable Tablets (FDA)
- Vitamins A, C, D and Fluoride (FDA)
Other brands
MVC-Fluoride, TRI-VIT With Fluoride, Tri-Vite Drops with Fluoride

