Multi-Vit with Fluoride Drops: Package Insert / Prescribing Info
Package insert / product label
Generic name: multi-vitamin with fluoride
Dosage form: oral solution/drops
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Feb 27, 2025.
On This Page
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 mL | ||
| Servings Per Container: 50 | ||
| Amount per serving | % Daily Value* | |
| Percent Daily Value (%DV) based on a 2,000 calorie diet. | ||
| Vitamin A | 1,500 IU | 60 |
| Vitamin C | 35 mg | 88 |
| Vitamin D | 400 IU | 100 |
| Vitamin E | 5 IU | 50 |
| Thiamine | 0.5 mg | 71 |
| Riboflavin | 0.6 mg | 75 |
| Niacin | 8 mg | 89 |
| Vitamin B6 | 0.4 mg | 57 |
| Vitamin B12 | 2 mcg | 67 |
| Fluoride (as Sodium Fluoride) | 0.5 mg | † |
Ingredients: Purified Water, Glycerin, Propylene Glycol, Ascorbic Acid, Polysorbate 80, Niacinamide, Sucralose, Sodium Hydroxide, D-Alpha-Tocopheryl Acid Succinate, Natural Grape Flavor, Caramel Color, Methyl Paraben, Sodium Benzoate, Sodium Fluoride, Vitamin A Palmitate, Riboflavin 5 Phosphate Sodium, Ferrous Sulfate, Thiamine Hydrochloride, Pyridoxine Hydrochloride, Sulfuric Acid, Cholecalciferol, Cyanocobalamin.
Warnings
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. IF OVERDOSAGE IS SUSPECTED, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY 1-800-222-1222.
SHAKE WELL BEFORE USING
Storage
Store in a cool, dry place at room temperature 20°-25°C (68°-77°F) away from heat and direct sunlight. Store in the original container.
Manufactured by:
Sancilio & Company, Inc.
3874 Fiscal Court
Riviera Beach, FL 33404
(800)SCI-8711
REV 001 8/28/17
Made in the USA
Related/similar drugs
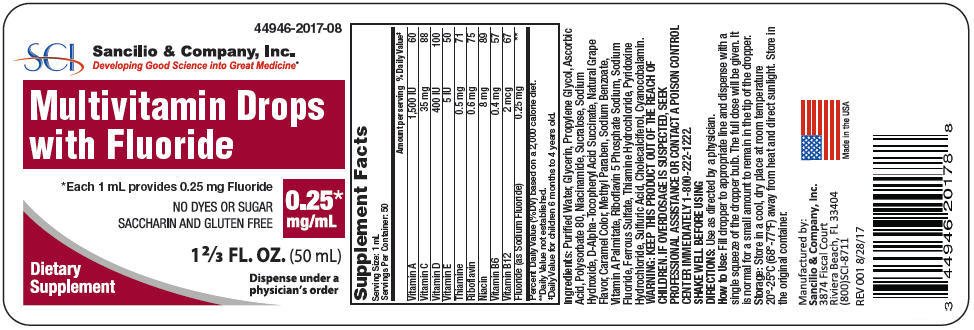
PRINCIPAL DISPLAY PANEL - 0.25 mg/mL Bottle Label
44946-2017-08
SCI
Sancilio & Company, Inc.
Developing Good Science into Great Medicine®
Multivitamin Drops
with Fluoride
*Each 1 mL provides 0.25 mg Fluoride
NO DYES OR SUGAR
SACCHARIN AND GLUTEN FREE
0.25*
mg/mL
1 ⅔ FL. OZ. (50 mL)
Dietary
Supplement
Dispense under a
physician's order
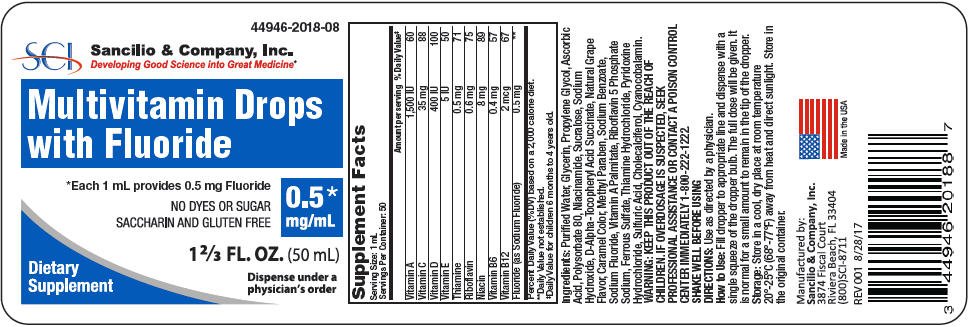
PRINCIPAL DISPLAY PANEL - 0.5 mg/mL Bottle Label
44946-2018-08
SCI
Sancilio & Company, Inc.
Developing Good Science into Great Medicine®
Multivitamin Drops
with Fluoride
*Each 1 mL provides 0.5 mg Fluoride
NO DYES OR SUGAR
SACCHARIN AND GLUTEN FREE
0.5*
mg/mL
1 ⅔ FL. OZ. (50 mL)
Dietary
Supplement
Dispense under a
physician's order
| MULTIVITAMIN DROPS WITH FLUORIDE
vitamin a palmitate, ascorbic acid, cholecalciferol, tocopherol, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, cyanocobalamin, and sodium fluoride liquid |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| MULTIVITAMIN DROPS WITH FLUORIDE
vitamin a palmitate, ascorbic acid, cholecalciferol, tocopherol, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, cyanocobalamin, and sodium fluoride liquid |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Sancilio & Company, Inc. (176681257) |
More about multivitamin with fluoride
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
- En español
Patient resources
- Multivitamins with fluoride drug information
- Pediatric Multivitamin Chewables with Fluoride
- Pediatric Multivitamin Drops with Fluoride
Professional resources
- Multi Vitamin Fluoride Drops prescribing information
- Multi Vitamin with Fluoride (FDA)
- MultiVit with Fluoride Chewable Tablets (FDA)
- Multivitamin with Fluoride Chewable Tablets (FDA)
- Vitamins A, C, D and Fluoride (FDA)
Other brands
MVC-Fluoride, TRI-VIT With Fluoride, Tri-Vite Drops with Fluoride

