Cervidil: Package Insert / Prescribing Info
Package insert / product label
Generic name: dinoprostone
Dosage form: vaginal insert
Drug class: Uterotonic agents
Medically reviewed by Drugs.com. Last updated on Jun 2, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
CERVIDIL® (dinoprostone) vaginal insert
Initial U.S. Approval: 1977
Indications and Usage for Cervidil
CERVIDIL is a prostaglandin analog indicated for the initiation and/or continuation of cervical ripening in pregnant women at or near term in whom there is a medical or obstetrical indication for the induction of labor. (1)
Cervidil Dosage and Administration
- Administer a single vaginal insert (10 mg) for up to 12 hours of use (approximately 0.3 mg of dinoprostone is released per hour) (2.1)
- Remove upon onset of active labor or 12 hours after insertion. (2.1)
- Carefully monitor for uterine activity, fetal status and the progression of cervical dilatation and effacement. (2.1)
- CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities (2.2).
- See full prescribing information for complete preparation, administration, and removal instructions. (2.2, 2.3)
Dosage Forms and Strengths
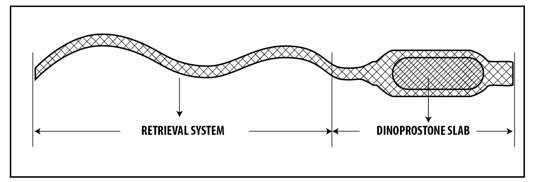
Vaginal Insert: 10 mg of dinoprostone contained within a knitted polyester pouch retrieval system. (3)
Contraindications
CERVIDIL is contraindicated for:
- Known hypersensitivity to prostaglandins (4)
- Evidence or clinical suspicion of fetal distress, where delivery is not imminent (4)
- Unexplained vaginal bleeding in the current pregnancy (4)
- Evidence or clinical suspicion of marked cephalopelvic disproportion (4)
- Contraindication to induction of labor (4)
- Concurrent use with intravenous oxytocic agents (4)
- History of previous cesarean section or other uterine surgery (such as myomectomy) (4)
- Conditions under which prolonged contraction of the uterus may be detrimental to fetal safety. (4)
- Six or more previous term pregnancies (4)
Warnings and Precautions
- Disseminated Intravascular Coagulation: Assess for evolving fibrinolysis in the immediate post-partum period. (5.2)
- Amniotic Fluid Embolism Syndrome: Carefully monitor patients for clinical signs of hypotension, hypoxemia and respiratory failure, DIC, coma or seizures. Provide supportive care. (5.3)
- Uterine Tachysystole and Uterine Hypersystole/Hypertonicity: Monitor uterine activity; remove vaginal insert. (5.4)
- History of Glaucoma: Consider non-prostaglandin cervical ripening procedures. (5.5)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 2 %) are uterine tachysystole without fetal distress, uterine tachysystole with fetal distress, and fetal distress without uterine tachysystole. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact FERRING PHARMACEUTICALS, INC. at 1 888-FERRING (1-888-337-7464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
Full Prescribing Information
1. Indications and Usage for Cervidil
CERVIDIL is indicated for the initiation and/or continuation of cervical ripening in pregnant women at or near term in whom there is a medical or obstetrical indication for the induction of labor.
2. Cervidil Dosage and Administration
2.1 Dosage Instructions
Administer one CERVIDIL insert (10 mg) intravaginally for use up to 12 hours (approximately 0.3 mg of dinoprostone is released per hour) [see Dosage and Administration (2.2)].
Monitor uterine activity, fetal status, and the progression of cervical dilatation and effacement with the use of CERVIDIL. Remove CERVIDIL 12 hours after insertion with the onset of active labor, prior to an amniotomy, occurrence of uterine tachysystole, uterine hypersystole/hypertonicity, or fetal distress [see Warnings and Precautions (5.4)]. Remove CERVIDIL at least 30 minutes prior to administering an oxytocic agent [see Warnings and Precautions (5.4) and Drug Interactions (7)].
2.2 Preparation and Administration Instructions
CERVIDIL should be administered only by trained obstetrical personnel in a hospital setting with appropriate obstetrical care facilities.
Preparation and Administration Instructions
- Keep CERVIDIL frozen until ready for use. Do not warm CERVIDIL prior to vaginal insertion.
- Tear open the individually-wrapped foil package containing CERVIDIL along the tear mark. Never open the package using scissors or other sharp objects because this may damage the knitted polyester retrieval system. Do not cut the retrieval system and do not use CERVIDIL unless its retrieval system is intact.
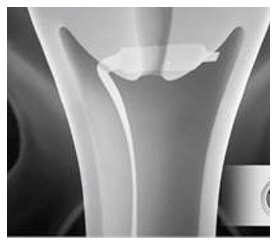
- Immediately after opening the package, insert CERVIDIL transversely, in the posterior fornix of the vagina (see Figure 1). If necessary, use a minimal amount of water-miscible lubricant to assist vaginal insertion. Do not permit excess contact or coating with the lubricant, as this could prevent release of dinoprostone from the vaginal insert. Insertion does not require sterile conditions.
- Tuck some of the excess retrieval system into the vagina to avoid movement of CERVIDIL away from the proper position; however, leave a small amount of the retrieval system outside the vagina to aid in retrieval.
- Instruct women to remain in a recumbent position during insertion of CERVIDIL and for 2 hours afterward. Women may be ambulatory 2 hours after insertion; however, ensure that the insert remains in place.
3. Dosage Forms and Strengths
Vaginal Insert: 10 mg of dinoprostone (release rate approximately 0.3 mg/hour up to 12 hours) in a hydrogel polymer. The insert is beige-colored, semi-opaque, thin, flat, and rectangular in shape with rounded corners, measuring 29 mm by 9.5 mm by 0.8 mm, contained within an off-white knitted polyester pouch retrieval system (see Figure 2).
4. Contraindications
CERVIDIL is contraindicated in patients with:
- Known hypersensitivity to prostaglandins [see Adverse Reactions (6.2)]
- Evidence or clinical suspicion of fetal distress where delivery is not imminent
- Unexplained vaginal bleeding in the current pregnancy
- Evidence or clinical suspicion of marked cephalopelvic disproportion
- Conditions for which induction of labor is contraindicated
- Conditions for which oxytocic drugs are contraindicated
- Previous cesarean section or other uterine surgery expected to affect uterine integrity (such as myomectomy)
- Conditions under which prolonged contraction of the uterus may be detrimental to fetal safety
- Concurrent use with intravenous oxytocic agents [see Warnings and Precautions (5.4) and Drug Interactions (7)]
- Six or more previous term pregnancies
5. Warnings and Precautions
5.1 For Hospital Use Only
CERVIDIL should be administered in a hospital setting with an obstetrical care facility.
5.2 Disseminated Intravascular Coagulation
CERVIDIL should be used with caution in women at high risk of postpartum disseminated intravascular coagulation (DIC). Physiologic or pharmacologic induction of labor, including the use of CERVIDIL, is associated with an increased risk of DIC during the postpartum period. Women aged 30 years or older, those with complications during pregnancy and those with a gestational age over 40 weeks have an increased risk of DIC during the postpartum period. As soon as possible, assess for an evolving fibrinolysis in the immediate post-partum period. Therapy consisting of prompt removal of the source of procoagulant material, replacement of depleted clotting factors and, in some cases, anti-coagulation with heparin should be instituted promptly.
5.3 Amniotic Fluid Embolism Syndrome
The use of dinoprostone-containing products, including CERVIDIL, can result in inadvertent disruption and subsequent embolization of antigenic tissue causing the development of Amniotic Fluid Embolism Syndrome, a rare and often fatal obstetric condition.
Carefully monitor patients for clinical signs of Amniotic Fluid Embolism Syndrome including hypotension, hypoxemia and respiratory failure, DIC, coma or seizures and provide supportive care as needed.
5.4 Uterine Tachysystole and Uterine Hypersystole/Hypertonicity
The use of CERVIDIL may cause uterine tachysystole with or without fetal heart rate changes (see Table 1). While using CERVIDIL, carefully monitor uterine activity, fetal status and the progression of cervical dilatation and effacement. Remove CERVIDIL with any evidence of uterine tachysystole, uterine hypersystole/hypertonicity, fetal distress, or if labor commences. CERVIDIL is contraindicated when prolonged contraction of the uterus is detrimental to fetal safety or uterine integrity, such as previous cesarean section or major uterine surgery, because of the risk of uterine rupture and obstetrical complications (e.g., need for hysterectomy and the occurrence of fetal or neonatal death). Prostaglandins, including CERVIDIL, may potentiate the effect of oxytocin [see Drug Interactions (7)]. Remove CERVIDIL at least 30 minutes before administration of an oxytocic agent is initiated and continue to carefully monitor uterine activity. Remove CERVIDIL prior to amniotomy or following rupture of membranes because the higher vaginal pH that occurs with rupture of membranes may result in higher release rate of dinoprostone.
6. Adverse Reactions/Side Effects
The following adverse reactions are described, or described in greater detail, in other sections:
- Disseminated Intravascular Coagulation [see Warnings and Precautions (5.2)]
- Amniotic Fluid Embolism [see Warnings and Precautions (5.3)]
- Uterine Tachysytole and Uterine Hypersystole/Hypertonicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In placebo-controlled trials of 658 pregnant women (320 CERVIDIL-treated women and 338 placebo-treated women), the following treatment related adverse reactions (see Table 1) occurred at an incidence greater than 2% (and greater than that reported in the placebo group) in the CERVIDIL group [see Clinical Studies (14)].
| Trials 1*and 2* | ||
| CERVIDIL (N=320) | Placebo (N=338) | |
| Uterine tachysystole with fetal distress | 2.8% | 0.3% |
| Uterine tachysystole without fetal distress | 4.7% | 0% |
| Fetal distress without uterine tachysystole - | 3.8% | 1.2% |
| Trial 3† | ||
| CERVIDIL (N=102) | Placebo (N=104) | |
| Uterine tachysystole with fetal distress | 2.9% | 0% |
| Uterine tachysystole -without fetal distress | 2% | 0% |
| Fetal distress without uterine tachysystole | 2.9% | 1% |
Drug related fever, nausea, vomiting, diarrhea, and abdominal pain occurred in less than 1% of CERVIDIL-treated patients.
In Trial 3 (with the retrieval system) cases of tachysystole uterine hyperstimulation reversed within 2 to 13 minutes of removal of CERVIDIL. Tocolytics were required in one of the five cases.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of CERVIDIL or other dinoprostone products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Disseminated Intravascular Coagulation
Cardiovascular disorders: Myocardial Infarction in women with a history of myocardial infarction
Immune system disorders: Hypersensitivity
Nervous system disorders: Headache
Pregnancy, puerperium and perinatal conditions: Amniotic fluid embolism
Reproductive system: reports of uterine rupture have been reported in association with use of CERVIDIL. Some required a hysterectomy and others resulted in subsequent fetal or neonatal death. Uterine hypertonus
Vascular disorders: Hypotension
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
CERVIDIL is indicated for the initiation and/or continuation of cervical ripening in pregnant women at or near term in whom there is a medical or obstetrical indication for the induction of labor. Fetal, neonatal, and maternal risks are discussed throughout the labeling. Limited available data with CERVIDIL use in pregnant women do not show a clear association with adverse developmental outcomes. Relevant animal reproduction data with dinoprostone is not available.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
When CERVIDIL was removed for fetal distress, there was a return to normal rhythm and there were no neonatal sequelae. Remove CERVIDIL in the event of persistent tachysystole with or without fetal heart rate changes, and follow established institutional protocols in management of patients.
8.2 Lactation
Risk Summary
Concomitant administration of CERVIDIL is not indicated in breastfeeding women. There is no information on the effects of maternal CERVIDIL administration on the breastfed child. Insufficient information is available on the effects of maternal CERVIDIL administration on milk production.
11. Cervidil Description
CERVIDIL (dinoprostone) vaginal insert contains dinoprostone, a prostaglandin analog. Each vaginal insert contains 10 mg of dinoprostone in 241 mg of a cross-linked polyethylene oxide/urethane polymer (hydrogel polymer) that is semi-opaque, beige colored, flat, rectangular in shape with rounded corners and measuring 29 mm by 9.5 mm by 0.8 mm. The vaginal insert is contained within a pouch of an off-white knitted polyester yarn retrieval system. When placed in a moist environment, the yarn absorbs water, swells, and releases the enclosed dinoprostone. The knitted polyester retrieval system has a long tape-like end that is designed to aid retrieval of CERVIDIL at the end of the dosing interval or earlier if clinically indicated. The finished product is a controlled-release formulation that has been found to release dinoprostone in vivo at a rate of approximately 0.3 mg per hour.
The chemical name for dinoprostone (known as prostaglandin E2 or PGE2) is 11α,15S-dihydroxy-9-oxo-prosta-5Z,13E-dien-1-oic acid and the structural formula is represented below:
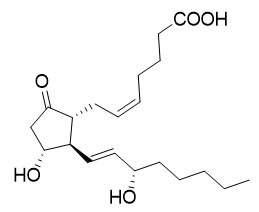
The molecular formula is C20H32O5 and its molecular weight is 352.47. Dinoprostone occurs as a white to off-white crystalline powder. It has a melting point within the range of 65° to 69°C. Dinoprostone is soluble in ethanol and in 25% ethanol in water.
12. Cervidil - Clinical Pharmacology
12.1 Cervidil Mechanism of Action
Dinoprostone is found in low concentrations in most tissues of the body and functions as a local hormone. In pregnancy, dinoprostone is secreted continuously by the fetal membranes and placenta and plays an important role in the final events leading to the initiation of labor including cervical ripening. Dinoprostone stimulates the production of prostaglandin F2α (PGF2α), which sensitizes the myometrium to endogenous or exogenously administered oxytocin. Available evidence indicates that dinoprostone, in the concentrations found during the early part of labor, plays an important role in cervical ripening without affecting uterine contractions.
In most patients, local effects of CERVIDIL on the cervix include changes in the tissue consistency, dilatation and effacement. Some women experience systemic effects, including uterine tachysystole, and uterine hypersystole/hypertonicity, as a result dinoprostone or PGF2α mediated sensitization of the myometrium to oxytocin [see Warnings and Precautions (5.4)].
12.3 Pharmacokinetics
The delivery rate of dinoprostone from CERVIDIL in vivo is approximately 0.3 mg/per hour over a period of 12 hours. Dinoprostone is metabolized in the tissues of synthesis with the half-life estimated to be 2.5 to 5 minutes. The rate limiting step for inactivation is regulated by the enzyme 15-hydroxyprostaglandin dehydrogenase (PGDH). Any dinoprostone that escapes local inactivation is cleared to the extent of 95% on the first pass through the pulmonary circulation.
No correlation could be established between the release of dinoprostone from CERVIDIL and plasma concentrations of metabolite of dinoprostone (PGEm). The relative contributions of endogenously and exogenously released dinoprostone to the plasma levels of the metabolite PGEm is not known.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity and fertility studies have not been conducted with dinoprostone. No evidence of mutagenicity has been observed with dinoprostone in the Unscheduled DNA Synthesis Assay, the Micronucleus Test, or the bacteria reverse mutation (Ames) test.
14. Clinical Studies
The effectiveness and safety of CERVIDIL for the induction of cervical ripening was evaluated in 658 pregnant women (320 CERVIDIL-treated women and 338 placebo-treated women) at or near term in three randomized, double-blind, placebo-controlled trials (Trials 1, 2, and 3). Efficacy outcomes included percentage with treatment success (defined as vaginal delivery within 12 hours, Bishop Score ≥ 6 in the 12-hour observation period, or ≥ 3 increase in the Bishop score in the 12-hour observation period), time to delivery, and time to onset of labor. Table 2 presents efficacy outcomes from Trials 1, 2, and 3.
| Primipara/Nullipara | Multipara | ||||
|---|---|---|---|---|---|
| Study # | CERVIDIL | Placebo | CERVIDIL | Placebo | P-Value |
|
|||||
| Treatment Success‡ | |||||
| Trial 1 (N=81) | 65% (n=26) | 28% (n=32) | 87% (n=16) | 29% (n=7) | <0.001 |
| Trial 2 (N=371) | 68% (n=111) | 24% (n=123) | 77% (n=65) | 24% (n=72) | <0.001 |
| Trial 3 (N=206) | 72% (n=60) | 48% (n=63) | 55% (n=42) | 41% (n=41) | 0.003 |
| Median Time to Delivery (hours) | |||||
| Trial 1 (N=81) | 25.7 (n=26) | 34.5 (n=32) | 12.3 (n=16) | 24.6 (n=7) | 0.001 |
| Trial 3 (N=206) | 25.5 (n=60) | 37.2 (n=63) | 20.8 (n=42) | 27.4 (n=41) | <0.001 |
| Median Time to Onset of Labor (hours) | |||||
| Trial 1 (N=81) | 12 (n=26) | 19.2 (n=32) | 6.9 (n=16) | 18.3 (n=7) | <0.001 |
16. How is Cervidil supplied
How Supplied
CERVIDIL vaginal insert contains 10 mg dinoprostone in a hydrogel polymer (NDC 55566-2800-1). The vaginal insert is beige-colored, semi-opaque, thin, flat, and rectangular in shape with rounded corners, measuring 29 mm by 9.5 mm by 0.8 mm, and is contained within an off-white knitted polyester pouch retrieval system.
CERVIDIL is enclosed in an aluminum/polyethylene pack.
Storage and Handling
Store in a freezer between -20°C and -10°C (-4°F and 14°F). CERVIDIL, enclosed in its aluminum/polyethylene pack, is stable when stored in a freezer for a period of three years. Vaginal inserts exposed to high humidity will absorb moisture from the air and thereby alter the release characteristics of dinoprostone.
17. Patient Counseling Information
Administration
Advise the woman to remain in the recumbent position for 2 hours following CERVIDIL insertion and to inform her health care provider immediately if CERVIDIL does not remain in place [see Dosage and Administration (2.2)].
Disseminated Intravascular Coagulation
Inform women that the use of CERVIDIL is associated with an increased risk of disseminated intravascular coagulation (DIC) during the postpartum period [see Warnings and Precautions (5.2)].
Amniotic Fluid Embolism Syndrome
Inform women that the use of CERVIDIL can result in inadvertent disruption and subsequent embolization of antigenic tissue causing the development of Amniotic Fluid Embolism Syndrome, a rare and often fatal obstetric condition [see Warnings and Precautions (5.3)].
Frequent or Prolonged Uterine Contractions
Inform women that the use of CERVIDIL may cause frequent or prolonged contractions [see Warnings and Precautions (5.4)]. This might result in disruption of blood flow through the placenta and to the fetus.
Glaucoma
Inform women that CERVIDIL can lead to raised intraocular pressure and constriction of pupils [see Warnings and Precautions (5.5)].
PRINCIPAL DISPLAY PANEL - 10 mg Pouch Carton
NDC 55566-2800-1
Cervidil®
DINOPROSTONE 10mg
VAGINAL INSERT
Contains: One Cervidil® Vaginal Insert containing
10 mg Dinoprostone in 241 mg hydrogel polymer
(cross-linked polyethylene oxide/urethane)
with polyester retrieval system.
Store in a freezer:
between -20°C and -10°C (-4°F and 14°F)
FERRING
PHARMACEUTICALS

| CERVIDIL
dinoprostone insert |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ferring Pharmaceuticals Inc. (103722955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ferring Controlled Therapeutics Ltd | 298229634 | manufacture(55566-2800) , pack(55566-2800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EUROAPI Hungary Ltd. | 402388457 | analysis(55566-2800) , api manufacture(55566-2800) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kyowa Pharma Chemical Co., Ltd. | 690852371 | api manufacture(55566-2800) | |
More about Cervidil (dinoprostone topical)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (18)
- Side effects
- Dosage information
- During pregnancy
- Drug class: uterotonic agents
- Breastfeeding
- En español